Self-inactivating gammaretroviral vectors for gene therapy of X-linked severe combined immunodeficiency
- PMID: 18180772
- PMCID: PMC6748866
- DOI: 10.1038/sj.mt.6300393
Self-inactivating gammaretroviral vectors for gene therapy of X-linked severe combined immunodeficiency
Abstract
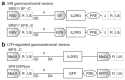
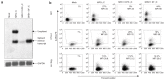
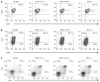
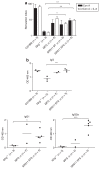
Gene therapy for X-linked severe combined immunodeficiency (SCID-X1) has proven highly effective for long-term restoration of immunity in human subjects. However, the development of lymphoproliferative complications due to dysregulated proto-oncogene expression has underlined the necessity for developing safer vector systems. To reduce the potential for insertional mutagenesis, we have evaluated the efficacy of self-inactivating (SIN) gammaretroviral vectors in cellular and in vivo models of SCID-X1. Vectors incorporating an internal human elongation factor-1alpha regulatory element were capable of fully restoring the lymphoid differentiation potential of gammac-deficient lineage negative cells. Multilineage lymphoid reconstitution of a murine model was achieved at a similar level to that achieved by a conventional long-terminal repeat (LTR)-regulated vector used in previous clinical trials. Functional proliferative responses to mitogenic stimuli were also restored, and serum immunoglobulin levels were normalized. The reduced mutagenic potential conferred by SIN vector configurations and alternative non-LTR-based regulatory elements, together with proven efficacy in correction of cellular defects provides an important platform for development of the next phase of clinical trials for SCID-X1.
Figures

Similar articles
-
Long-term persistence of a polyclonal T cell repertoire after gene therapy for X-linked severe combined immunodeficiency.Sci Transl Med. 2011 Aug 24;3(97):97ra79. doi: 10.1126/scitranslmed.3002715. Sci Transl Med. 2011. PMID: 21865537 Clinical Trial.
-
Gene Therapy for X-Linked Severe Combined Immunodeficiency: Where Do We Stand?Hum Gene Ther. 2016 Feb;27(2):108-16. doi: 10.1089/hum.2015.137. Hum Gene Ther. 2016. PMID: 26790362 Free PMC article. Review.
-
Gene therapy model of X-linked severe combined immunodeficiency using a modified foamy virus vector.PLoS One. 2013 Aug 21;8(8):e71594. doi: 10.1371/journal.pone.0071594. eCollection 2013. PLoS One. 2013. PMID: 23990961 Free PMC article.
-
A modified γ-retrovirus vector for X-linked severe combined immunodeficiency.N Engl J Med. 2014 Oct 9;371(15):1407-17. doi: 10.1056/NEJMoa1404588. N Engl J Med. 2014. PMID: 25295500 Free PMC article. Clinical Trial.
-
Immune Reconstitution After Gene Therapy Approaches in Patients With X-Linked Severe Combined Immunodeficiency Disease.Front Immunol. 2020 Nov 27;11:608653. doi: 10.3389/fimmu.2020.608653. eCollection 2020. Front Immunol. 2020. PMID: 33329605 Free PMC article. Review.
Cited by
-
Long Terminal Repeats of Gammaretroviruses Retain Stable Expression after Integration Retargeting.Viruses. 2024 Sep 25;16(10):1518. doi: 10.3390/v16101518. Viruses. 2024. PMID: 39459853 Free PMC article.
-
Molecular Mechanisms in Pathophysiology of Mucopolysaccharidosis and Prospects for Innovative Therapy.Int J Mol Sci. 2024 Jan 17;25(2):1113. doi: 10.3390/ijms25021113. Int J Mol Sci. 2024. PMID: 38256186 Free PMC article. Review.
-
From bench to bedside: preclinical evaluation of a self-inactivating gammaretroviral vector for the gene therapy of X-linked chronic granulomatous disease.Hum Gene Ther Clin Dev. 2013 Jun;24(2):86-98. doi: 10.1089/humc.2013.019. Hum Gene Ther Clin Dev. 2013. PMID: 23845071 Free PMC article.
-
NOX Inhibitors: From Bench to Naxibs to Bedside.Handb Exp Pharmacol. 2021;264:145-168. doi: 10.1007/164_2020_387. Handb Exp Pharmacol. 2021. PMID: 32780287
-
Improving TCR Gene Therapy for Treatment of Haematological Malignancies.Adv Hematol. 2012;2012:404081. doi: 10.1155/2012/404081. Epub 2012 Jan 26. Adv Hematol. 2012. PMID: 22319532 Free PMC article.
References
-
- Fischer A, Le Deist F, Hacein-Bey-Abina S, André-Schmutz I, Basile Gde S, de Villartay JP, et al. Severe combined immunodeficiency. A model disease for molecular immunology and therapy. Immunol Rev. 2005;203:98–109. - PubMed
-
- Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–655. - PubMed
-
- White H, Thrasher A, Veys P, Kinnon C, Gaspar HB. Intrinsic defects of B cell function in X-linked severe combined immunodeficiency. Eur J Immunol. 2000;30:732–737. - PubMed
-
- Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts JL, et al. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340:508–516. - PubMed
-
- Antoine C, Muller S, Cant A, Cavazzana-Calvo M, Veys P, Vossen J, et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968–99. Lancet. 2003;361:553–560. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

