Alpha-synuclein-induced aggregation of cytoplasmic vesicles in Saccharomyces cerevisiae
- PMID: 18172022
- PMCID: PMC2262993
- DOI: 10.1091/mbc.e07-08-0827
Alpha-synuclein-induced aggregation of cytoplasmic vesicles in Saccharomyces cerevisiae
Erratum in
- Mol Biol Cell. 2008 May;19(5):2348
Abstract
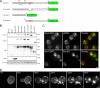
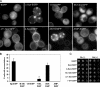
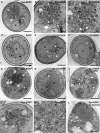
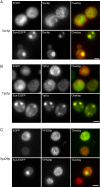
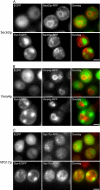
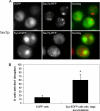
Aggregated alpha-synuclein (alpha-syn) fibrils form Lewy bodies (LBs), the signature lesions of Parkinson's disease (PD) and related synucleinopathies, but the pathogenesis and neurodegenerative effects of LBs remain enigmatic. Recent studies have shown that when overexpressed in Saccharomyces cerevisiae, alpha-syn localizes to plasma membranes and forms cytoplasmic accumulations similar to human alpha-syn inclusions. However, the exact nature, composition, temporal evolution, and underlying mechanisms of yeast alpha-syn accumulations and their relevance to human synucleinopathies are unknown. Here we provide ultrastructural evidence that alpha-syn accumulations are not comprised of LB-like fibrils, but are associated with clusters of vesicles. Live-cell imaging showed alpha-syn initially localized to the plasma membrane and subsequently formed accumulations in association with vesicles. Imaging of truncated and mutant forms of alpha-syn revealed the molecular determinants and vesicular trafficking pathways underlying this pathological process. Because vesicular clustering is also found in LB-containing neurons of PD brains, alpha-syn-mediated vesicular accumulation in yeast represents a model system to study specific aspects of neurodegeneration in PD and related synucleinopathies.
Figures

Similar articles
-
The Parkinson's disease protein alpha-synuclein disrupts cellular Rab homeostasis.Proc Natl Acad Sci U S A. 2008 Jan 8;105(1):145-50. doi: 10.1073/pnas.0710685105. Epub 2007 Dec 27. Proc Natl Acad Sci U S A. 2008. PMID: 18162536 Free PMC article.
-
Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells.Proc Natl Acad Sci U S A. 2009 Nov 24;106(47):20051-6. doi: 10.1073/pnas.0908005106. Epub 2009 Nov 5. Proc Natl Acad Sci U S A. 2009. PMID: 19892735 Free PMC article.
-
Alpha-Synuclein and the Endolysosomal System in Parkinson's Disease: Guilty by Association.Biomolecules. 2021 Sep 9;11(9):1333. doi: 10.3390/biom11091333. Biomolecules. 2021. PMID: 34572546 Free PMC article. Review.
-
Alteration of Structure and Aggregation of α-Synuclein by Familial Parkinson's Disease Associated Mutations.Curr Protein Pept Sci. 2017;18(7):656-676. doi: 10.2174/1389203717666160314151706. Curr Protein Pept Sci. 2017. PMID: 26972727 Review.
-
Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models.Science. 2006 Jul 21;313(5785):324-8. doi: 10.1126/science.1129462. Epub 2006 Jun 22. Science. 2006. PMID: 16794039 Free PMC article.
Cited by
-
Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis.Neuron. 2010 Jan 14;65(1):66-79. doi: 10.1016/j.neuron.2009.12.023. Neuron. 2010. PMID: 20152114 Free PMC article.
-
{alpha}-synuclein and its A30P mutant affect actin cytoskeletal structure and dynamics.Mol Biol Cell. 2009 Aug;20(16):3725-39. doi: 10.1091/mbc.e08-03-0302. Epub 2009 Jun 24. Mol Biol Cell. 2009. PMID: 19553474 Free PMC article.
-
Therapeutic genetic variation revealed in diverse Hsp104 homologs.Elife. 2020 Dec 15;9:e57457. doi: 10.7554/eLife.57457. Elife. 2020. PMID: 33319748 Free PMC article.
-
The Role of Alpha-Synuclein and Other Parkinson's Genes in Neurodevelopmental and Neurodegenerative Disorders.Int J Mol Sci. 2020 Aug 10;21(16):5724. doi: 10.3390/ijms21165724. Int J Mol Sci. 2020. PMID: 32785033 Free PMC article. Review.
-
Endogenous alpha-synuclein monomers, oligomers and resulting pathology: let's talk about the lipids in the room.NPJ Parkinsons Dis. 2019 Nov 12;5:23. doi: 10.1038/s41531-019-0095-3. eCollection 2019. NPJ Parkinsons Dis. 2019. PMID: 31728405 Free PMC article. Review.
References
-
- Abeliovich A., et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
-
- Chandra S., Gallardo G., Fernandez-Chacon R., Schluter O. M., Sudhof T. C. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. - PubMed
-
- Chartier-Harlin M. C., et al. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–1169. - PubMed
-
- Cole N. B., Murphy D. D., Grider T., Rueter S., Brasaemle D., Nussbaum R. L. Lipid droplet binding and oligomerization properties of the Parkinson's disease protein alpha-synuclein. J. Biol. Chem. 2002;277:6344–6352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

