Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons
- PMID: 18167537
- PMCID: PMC2147051
- DOI: 10.1371/journal.pone.0001376
Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons
Abstract
Background: The neuropathology of Parkinson's disease (PD) includes loss of dopaminergic neurons in the substantia nigra, nitrated alpha-synuclein (N-alpha-Syn) enriched intraneuronal inclusions or Lewy bodies and neuroinflammation. While the contribution of innate microglial inflammatory activities to disease are known, evidence for how adaptive immune mechanisms may affect the course of PD remains obscure. We reasoned that PD-associated oxidative protein modifications create novel antigenic epitopes capable of peripheral adaptive T cell responses that could affect nigrostriatal degeneration.
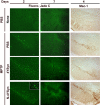
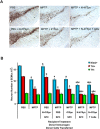
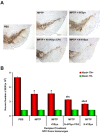
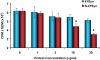
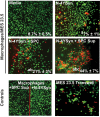
Methods and findings: Nitrotyrosine (NT)-modified alpha-Syn was detected readily in cervical lymph nodes (CLN) from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) intoxicated mice. Antigen-presenting cells within the CLN showed increased surface expression of major histocompatibility complex class II, initiating the molecular machinery necessary for efficient antigen presentation. MPTP-treated mice produced antibodies to native and nitrated alpha-Syn. Mice immunized with the NT-modified C-terminal tail fragment of alpha-Syn, but not native protein, generated robust T cell proliferative and pro-inflammatory secretory responses specific only for the modified antigen. T cells generated against the nitrated epitope do not respond to the unmodified protein. Mice deficient in T and B lymphocytes were resistant to MPTP-induced neurodegeneration. Transfer of T cells from mice immunized with N-alpha-Syn led to a robust neuroinflammatory response with accelerated dopaminergic cell loss.
Conclusions: These data show that NT modifications within alpha-Syn, can bypass or break immunological tolerance and activate peripheral leukocytes in draining lymphoid tissue. A novel mechanism for disease is made in that NT modifications in alpha-Syn induce adaptive immune responses that exacerbate PD pathobiology. These results have implications for both the pathogenesis and treatment of this disabling neurodegenerative disease.
Conflict of interest statement
Figures


Similar articles
-
Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson's disease.J Immunol. 2010 Mar 1;184(5):2261-71. doi: 10.4049/jimmunol.0901852. Epub 2010 Jan 29. J Immunol. 2010. PMID: 20118279 Free PMC article.
-
Targeting of the class II transactivator attenuates inflammation and neurodegeneration in an alpha-synuclein model of Parkinson's disease.J Neuroinflammation. 2018 Aug 30;15(1):244. doi: 10.1186/s12974-018-1286-2. J Neuroinflammation. 2018. PMID: 30165873 Free PMC article.
-
Dose-related biphasic effect of the Parkinson's disease neurotoxin MPTP, on the spread, accumulation, and toxicity of α-synuclein.Neurotoxicology. 2021 May;84:41-52. doi: 10.1016/j.neuro.2021.02.001. Epub 2021 Feb 4. Neurotoxicology. 2021. PMID: 33549656
-
Leveraging the preformed fibril model to distinguish between alpha-synuclein inclusion- and nigrostriatal degeneration-associated immunogenicity.Neurobiol Dis. 2022 Sep;171:105804. doi: 10.1016/j.nbd.2022.105804. Epub 2022 Jun 25. Neurobiol Dis. 2022. PMID: 35764290 Free PMC article. Review.
-
Calpain activation and progression of inflammatory cycles in Parkinson's disease.Front Biosci (Landmark Ed). 2022 Jan 13;27(1):20. doi: 10.31083/j.fbl2701020. Front Biosci (Landmark Ed). 2022. PMID: 35090325 Free PMC article. Review.
Cited by
-
Endoplasmic Reticulum Stress Interacts With Inflammation in Human Diseases.J Cell Physiol. 2016 Feb;231(2):288-94. doi: 10.1002/jcp.25098. J Cell Physiol. 2016. PMID: 26201832 Free PMC article. Review.
-
α-Synuclein vaccination modulates regulatory T cell activation and microglia in the absence of brain pathology.J Neuroinflammation. 2016 Apr 7;13(1):74. doi: 10.1186/s12974-016-0532-8. J Neuroinflammation. 2016. PMID: 27055651 Free PMC article.
-
The prion hypothesis of Parkinson's disease.Curr Neurol Neurosci Rep. 2015 May;15(5):28. doi: 10.1007/s11910-015-0549-x. Curr Neurol Neurosci Rep. 2015. PMID: 25868519
-
Triptolide Inhibits Preformed Fibril-Induced Microglial Activation by Targeting the MicroRNA155-5p/SHIP1 Pathway.Oxid Med Cell Longev. 2019 Apr 28;2019:6527638. doi: 10.1155/2019/6527638. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31182996 Free PMC article.
-
Abnormal subpopulations of peripheral blood lymphocytes are involved in Parkinson's disease.Ann Transl Med. 2019 Nov;7(22):637. doi: 10.21037/atm.2019.10.105. Ann Transl Med. 2019. PMID: 31930038 Free PMC article.
References
-
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. - PubMed
-
- Klockgether T. Parkinson's disease: clinical aspects. Cell Tissue Res. 2004;318:115–120. - PubMed
-
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. - PubMed
-
- Sidhu A, Wersinger C, Vernier P. Does alpha-synuclein modulate dopaminergic synaptic content and tone at the synapse? FASEB J. 2004;18:637–647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS11766/NS/NINDS NIH HHS/United States
- NS36136/NS/NINDS NIH HHS/United States
- P01 NS043985/NS/NINDS NIH HHS/United States
- R01 AG021617/AG/NIA NIH HHS/United States
- P50 NS038370/NS/NINDS NIH HHS/United States
- NS38370/NS/NINDS NIH HHS/United States
- ES013177/ES/NIEHS NIH HHS/United States
- AG021617/AG/NIA NIH HHS/United States
- R21 NS049264/NS/NINDS NIH HHS/United States
- MH64570/MH/NIMH NIH HHS/United States
- NS049264/NS/NINDS NIH HHS/United States
- NS43985/NS/NINDS NIH HHS/United States
- R21 ES013177/ES/NIEHS NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- NS007488/NS/NINDS NIH HHS/United States
- P01 NS011766/NS/NINDS NIH HHS/United States
- T32 NS007488/NS/NINDS NIH HHS/United States
- R01 NS042269/NS/NINDS NIH HHS/United States
- NS42269/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

