Heterogeneity in EGF-binding affinities arises from negative cooperativity in an aggregating system
- PMID: 18165319
- PMCID: PMC2224169
- DOI: 10.1073/pnas.0707080105
Heterogeneity in EGF-binding affinities arises from negative cooperativity in an aggregating system
Erratum in
- Proc Natl Acad Sci U S A. 2008 Jul 1;105(26):9129
Abstract
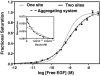
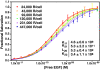
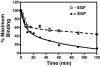
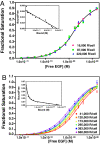
Scatchard analysis of the binding of EGF to its receptor yields concave up plots that indicate the presence of two classes of binding sites. However, how two independent classes of sites arise from the expression of a single EGF receptor protein has never been adequately explained. Using a new analytical approach involving the simultaneous fitting of binding isotherms from cells expressing increasing levels of EGF receptors, we show that (125)I-EGF-binding data can be completely explained by a model involving negative cooperativity in an aggregating system. This approach provides an experimentally determined value for the monomer-dimer equilibrium constant, which, for wild-type EGF receptors, corresponds to approximately 50,000 receptors per cell. Therefore, changes in receptor expression within the physiological range can modulate the outcome of a signaling stimulus. Analysis of the L680N-EGF receptor mutant, in which the formation of asymmetric kinase domain dimers is blocked, indicates that the kinase dimers make a substantial energetic contribution to the ligand-independent association of EGF receptor monomers, but are not necessary for negative cooperativity. The model accurately predicts the behavior of receptor mutants, such as the dimerization-defective Y246D-EGF receptor, which exhibit a single class of binding sites and provides a framework for understanding secondary dimer formation and lateral signaling in the EGF receptor family.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
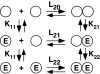
Similar articles
-
The membrane-proximal intracellular domain of the epidermal growth factor receptor underlies negative cooperativity in ligand binding.J Biol Chem. 2011 Dec 30;286(52):45146-55. doi: 10.1074/jbc.M111.274175. Epub 2011 Nov 8. J Biol Chem. 2011. PMID: 22069315 Free PMC article.
-
Two EGF molecules contribute additively to stabilization of the EGFR dimer.EMBO J. 1997 Jan 15;16(2):281-94. doi: 10.1093/emboj/16.2.281. EMBO J. 1997. PMID: 9029149 Free PMC article.
-
The tethering arm of the EGF receptor is required for negative cooperativity and signal transduction.J Biol Chem. 2011 Jan 14;286(2):1545-55. doi: 10.1074/jbc.M110.182899. Epub 2010 Nov 3. J Biol Chem. 2011. PMID: 21047778 Free PMC article.
-
The multiple origins of cooperativity in binding to multi-site lattices.FEBS Lett. 1996 Nov 11;397(1):1-6. doi: 10.1016/s0014-5793(96)01020-4. FEBS Lett. 1996. PMID: 8941702 Review.
-
Proteomics and models for enzyme cooperativity.J Biol Chem. 2002 Dec 6;277(49):46841-4. doi: 10.1074/jbc.R200014200. Epub 2002 Aug 19. J Biol Chem. 2002. PMID: 12189158 Review. No abstract available.
Cited by
-
A structural perspective on the regulation of the epidermal growth factor receptor.Annu Rev Biochem. 2015;84:739-64. doi: 10.1146/annurev-biochem-060614-034402. Epub 2015 Jan 26. Annu Rev Biochem. 2015. PMID: 25621509 Free PMC article. Review.
-
Direct visualization of single-molecule membrane protein interactions in living cells.PLoS Biol. 2018 Dec 13;16(12):e2006660. doi: 10.1371/journal.pbio.2006660. eCollection 2018 Dec. PLoS Biol. 2018. PMID: 30543635 Free PMC article.
-
Quantifying the strength of heterointeractions among receptor tyrosine kinases from different subfamilies: Implications for cell signaling.J Biol Chem. 2020 Jul 17;295(29):9917-9933. doi: 10.1074/jbc.RA120.013639. Epub 2020 May 27. J Biol Chem. 2020. PMID: 32467228 Free PMC article.
-
Human epidermal growth factor receptor (EGFR) aligned on the plasma membrane adopts key features of Drosophila EGFR asymmetry.Mol Cell Biol. 2011 Jun;31(11):2241-52. doi: 10.1128/MCB.01431-10. Epub 2011 Mar 28. Mol Cell Biol. 2011. PMID: 21444717 Free PMC article.
-
Mechanistic insights into the activation of oncogenic forms of EGF receptor.Nat Struct Mol Biol. 2011 Nov 20;18(12):1388-93. doi: 10.1038/nsmb.2168. Nat Struct Mol Biol. 2011. PMID: 22101934 Free PMC article.
References
-
- Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam AW, Lee J, Yarden Y, Libermann TA, Schlessinger J, et al. Nature. 1984;309:418–425. - PubMed
-
- Yarden Y, Schlessinger J. Biochemistry. 1987;26:1443–1451. - PubMed
-
- Ferguson KM, Berger MB, Mendrola JM, Cho H-S, Leahy DJ, Lemmon MA. Mol Cell. 2003;11:507–517. - PubMed
-
- Garrett TPJ, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, Zhu H-J, Walker F, Frenkel MJ, Hoyne PA, et al. Cell. 2002;110:763–773. - PubMed
-
- Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim J-H, Saito K, Sakamoto A, Inoue M, Shirouzu M, Yokoyama S. Cell. 2002;110:775–787. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

