NADPH oxidases in lung biology and pathology: host defense enzymes, and more
- PMID: 18164271
- PMCID: PMC2323509
- DOI: 10.1016/j.freeradbiomed.2007.11.016
NADPH oxidases in lung biology and pathology: host defense enzymes, and more
Abstract
The deliberate production of reactive oxygen species (ROS) by phagocyte NADPH oxidase is widely appreciated as a critical component of antimicrobial host defense. Recently, additional homologs of NADPH oxidase (NOX) have been discovered throughout the animal and plant kingdoms, which appear to possess diverse functions in addition to host defense, in cell proliferation, differentiation, and in regulation of gene expression. Several of these NOX homologs are also expressed within the respiratory tract, where they participate in innate host defense as well as in epithelial and inflammatory cell signaling and gene expression, and fibroblast and smooth muscle cell proliferation, in response to bacterial or viral infection and environmental stress. Inappropriate expression or activation of NOX/DUOX during various lung pathologies suggests their specific involvement in respiratory disease. This review summarizes the current state of knowledge regarding the general functional properties of mammalian NOX enzymes, and their specific importance in respiratory tract physiology and pathology.
Figures
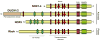
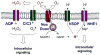


Similar articles
-
The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology.Physiol Rev. 2007 Jan;87(1):245-313. doi: 10.1152/physrev.00044.2005. Physiol Rev. 2007. PMID: 17237347 Review.
-
Role of Nox family NADPH oxidases in host defense.Antioxid Redox Signal. 2006 Sep-Oct;8(9-10):1549-61. doi: 10.1089/ars.2006.8.1549. Antioxid Redox Signal. 2006. PMID: 16987010 Review.
-
[The Nox/Duox family of ROS-generating NADPH oxidases].Med Sci (Paris). 2006 Nov;22(11):953-9. doi: 10.1051/medsci/20062211953. Med Sci (Paris). 2006. PMID: 17101097 Review. French.
-
Mucosal reactive oxygen species are required for antiviral response: role of Duox in influenza a virus infection.Antioxid Redox Signal. 2014 Jun 10;20(17):2695-709. doi: 10.1089/ars.2013.5353. Epub 2013 Oct 15. Antioxid Redox Signal. 2014. PMID: 24128054
-
Lung epithelial NOX/DUOX and respiratory virus infections.Clin Sci (Lond). 2015 Mar;128(6):337-47. doi: 10.1042/CS20140321. Clin Sci (Lond). 2015. PMID: 25456319 Review.
Cited by
-
NOX enzymes: potential target for the treatment of acute lung injury.Cell Mol Life Sci. 2012 Jul;69(14):2373-85. doi: 10.1007/s00018-012-1013-6. Epub 2012 May 13. Cell Mol Life Sci. 2012. PMID: 22581364 Free PMC article. Review.
-
DUOX1 mediates persistent epithelial EGFR activation, mucous cell metaplasia, and airway remodeling during allergic asthma.JCI Insight. 2016 Nov 3;1(18):e88811. doi: 10.1172/jci.insight.88811. JCI Insight. 2016. PMID: 27812543 Free PMC article.
-
Hyperoxia impairs postnatal alveolar epithelial development via NADPH oxidase in newborn mice.Am J Physiol Lung Cell Mol Physiol. 2009 Jul;297(1):L134-42. doi: 10.1152/ajplung.00112.2009. Epub 2009 May 1. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19411313 Free PMC article.
-
Dual oxidase 2 in lung epithelia is essential for hyperoxia-induced acute lung injury in mice.Antioxid Redox Signal. 2014 Nov 1;21(13):1803-18. doi: 10.1089/ars.2013.5677. Epub 2014 Jun 26. Antioxid Redox Signal. 2014. PMID: 24766345 Free PMC article.
-
Dual oxidase 2 bidirectional promoter polymorphisms confer differential immune responses in airway epithelia.Am J Respir Cell Mol Biol. 2012 Oct;47(4):484-90. doi: 10.1165/rcmb.2012-0037OC. Epub 2012 May 16. Am J Respir Cell Mol Biol. 2012. PMID: 22592922 Free PMC article.
References
-
- Geiszt M, Leto TL. The Nox family of NAD(P)H oxidases: host defense and beyond. J Biol Chem. 2004;279(50):51715–8. - PubMed
-
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4(3):181–9. - PubMed
-
- Jones RD, Hancock JT, Morice AH. NADPH oxidase: a universal oxygen sensor? Free Radic Biol Med. 2000;29(5):416–24. - PubMed
-
- Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397(2):342–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

