Beta-catenin expression results in p53-independent DNA damage and oncogene-induced senescence in prelymphomagenic thymocytes in vivo
- PMID: 18160717
- PMCID: PMC2258783
- DOI: 10.1128/MCB.01360-07
Beta-catenin expression results in p53-independent DNA damage and oncogene-induced senescence in prelymphomagenic thymocytes in vivo
Abstract
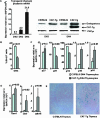
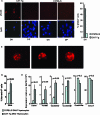
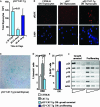
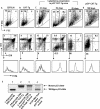
The expression of beta-catenin, a potent oncogene, is causally linked to tumorigenesis. Therefore, it was surprising that the transgenic expression of oncogenic beta-catenin in thymocytes resulted in thymic involution instead of lymphomagenesis. In this report, we demonstrate that this is because the expression of oncogenic beta-catenin induces DNA damage, growth arrest, oncogene-induced senescence (OIS), and apoptosis of immature thymocytes. In p53-deficient mice, the expression of oncogenic beta-catenin still results in DNA damage and OIS, but the thymocytes survive and eventually progress to thymic lymphoma. beta-Catenin-induced thymic lymphomas are distinct from lymphomas that arise in p53(-/-) mice. They are CD4(-) CD8(-), while p53-dependent lymphomas are largely CD4(+) CD8(+), and they develop at an earlier age and in the absence of c-Myc expression or Notch1 signaling. Thus, we report that oncogenic beta-catenin-induced, p53-independent growth arrest and OIS and p53-dependent apoptosis protect developing thymocytes from transformation by oncogenic beta-catenin.
Figures


Similar articles
-
Molecular basis for the tissue specificity of β-catenin oncogenesis.Oncogene. 2013 Apr 11;32(15):1901-9. doi: 10.1038/onc.2012.215. Epub 2012 Jun 11. Oncogene. 2013. PMID: 22689057 Free PMC article.
-
Deletion of Irf5 protects hematopoietic stem cells from DNA damage-induced apoptosis and suppresses γ-irradiation-induced thymic lymphomagenesis.Oncogene. 2014 Jun 19;33(25):3288-97. doi: 10.1038/onc.2013.295. Epub 2013 Aug 5. Oncogene. 2014. PMID: 23912454
-
p53 and thymic 'death by neglect': thymic epithelial cell-induced apoptosis of CD4+8+ thymocytes is p53-independent.Cell Death Differ. 2000 Mar;7(3):241-9. doi: 10.1038/sj.cdd.4400657. Cell Death Differ. 2000. PMID: 10745269
-
DNA-damaging agents induce both p53-dependent and p53-independent apoptosis in immature thymocytes.Mol Pharmacol. 1996 Oct;50(4):900-11. Mol Pharmacol. 1996. PMID: 8863836
-
Metabolic alterations accompanying oncogene-induced senescence.Mol Cell Oncol. 2014 Dec 23;1(3):e963481. doi: 10.4161/23723548.2014.963481. eCollection 2014 Jul-Sep. Mol Cell Oncol. 2014. PMID: 27308349 Free PMC article. Review.
Cited by
-
Wnt activity and basal niche position sensitize intestinal stem and progenitor cells to DNA damage.EMBO J. 2015 Mar 4;34(5):624-40. doi: 10.15252/embj.201490700. Epub 2015 Jan 21. EMBO J. 2015. PMID: 25609789 Free PMC article.
-
Molecular basis for the tissue specificity of β-catenin oncogenesis.Oncogene. 2013 Apr 11;32(15):1901-9. doi: 10.1038/onc.2012.215. Epub 2012 Jun 11. Oncogene. 2013. PMID: 22689057 Free PMC article.
-
Wnt/β-catenin signaling induces the aging of mesenchymal stem cells through promoting the ROS production.Mol Cell Biochem. 2013 Feb;374(1-2):13-20. doi: 10.1007/s11010-012-1498-1. Epub 2012 Nov 3. Mol Cell Biochem. 2013. PMID: 23124852
-
Wnt/β-catenin signaling induces the aging of mesenchymal stem cells through the DNA damage response and the p53/p21 pathway.PLoS One. 2011;6(6):e21397. doi: 10.1371/journal.pone.0021397. Epub 2011 Jun 21. PLoS One. 2011. PMID: 21712954 Free PMC article.
-
Novel ARF/p53-independent senescence pathways in cancer repression.J Mol Med (Berl). 2011 Sep;89(9):857-67. doi: 10.1007/s00109-011-0766-y. Epub 2011 May 19. J Mol Med (Berl). 2011. PMID: 21594579 Free PMC article. Review.
References
-
- Bartkova, J., N. Rezaei, M. Liontos, P. Karakaidos, D. Kletsas, N. Issaeva, L. V. Vassiliou, E. Kolettas, K. Niforou, V. C. Zoumpourlis, M. Takaoka, H. Nakagawa, F. Tort, K. Fugger, F. Johansson, M. Sehested, C. L. Andersen, L. Dyrskjot, T. Orntoft, J. Lukas, C. Kittas, T. Helleday, T. D. Halazonetis, J. Bartek, and V. G. Gorgoulis. 2006. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444633-637. - PubMed
-
- Braig, M., S. Lee, C. Loddenkemper, C. Rudolph, A. H. Peters, B. Schlegelberger, H. Stein, B. Dorken, T. Jenuwein, and C. A. Schmitt. 2005. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436660-665. - PubMed
-
- Campisi, J. 2001. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 11S27-S31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
