The role of the FH1 domain and profilin in formin-mediated actin-filament elongation and nucleation
- PMID: 18160294
- PMCID: PMC3712528
- DOI: 10.1016/j.cub.2007.11.062
The role of the FH1 domain and profilin in formin-mediated actin-filament elongation and nucleation
Erratum in
- Curr Biol. 2008 Feb 12;18(3):233. Paul, Aditya [corrected to Paul, Aditya S]; Pollard, Thomas [corrected to Pollard, Thomas D]
Abstract
Background: Formin proteins nucleate actin filaments de novo and stay associated with the growing barbed end. Whereas the formin-homology (FH) 2 domains mediate processive association, the FH1 domains-in concert with the actin-monomer-binding protein profilin-increase the rate of barbed-end elongation. The mechanism by which this effect is achieved is not well understood.
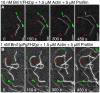
Results: We used total internal reflection fluorescence microscopy to measure the effect of profilin on the elongation of single actin filaments associated with FH1FH2 constructs (derived from the formin Bni1p from S. cerevisiae) with FH1 domains containing one to eight profilin-binding polyproline tracks. Over a large range of profilin concentrations (0.5-25 microM), the rate of barbed-end elongation increases with the number of polyproline tracks in the FH1 domain. The binding of profilin-actin to the FH1 domain is the rate-limiting step (up to rates of at least 88 s(-1)) in FH1-mediated transfer of actin subunits to the barbed end. Dissociation of formins from barbed ends growing in the presence of profilin is proportional to the elongation rate. Profilin profoundly inhibits nucleation by FH2 and FH1FH2 constructs, but profilin-actin bound to FH1 might contribute weakly to nucleation.
Conclusions: To achieve fast elongation, formin FH1 domains bind profilin-actin complexes and deliver them rapidly to the barbed end associated with the FH2 domain. Because subunit addition promotes dissociation of FH2 domains from growing barbed ends, FH2 domains must pass through a state that is prone to dissociation during each cycle of actin subunit addition coupled to formin translocation.
Figures






Similar articles
-
Review of the mechanism of processive actin filament elongation by formins.Cell Motil Cytoskeleton. 2009 Aug;66(8):606-17. doi: 10.1002/cm.20379. Cell Motil Cytoskeleton. 2009. PMID: 19459187 Free PMC article. Review.
-
Competition for delivery of profilin-actin to barbed ends limits the rate of formin-mediated actin filament elongation.J Biol Chem. 2020 Apr 3;295(14):4513-4525. doi: 10.1074/jbc.RA119.012000. Epub 2020 Feb 19. J Biol Chem. 2020. PMID: 32075907 Free PMC article.
-
Determinants of Formin Homology 1 (FH1) domain function in actin filament elongation by formins.J Biol Chem. 2012 Mar 2;287(10):7812-20. doi: 10.1074/jbc.M111.322958. Epub 2012 Jan 14. J Biol Chem. 2012. PMID: 22247555 Free PMC article.
-
Energetic requirements for processive elongation of actin filaments by FH1FH2-formins.J Biol Chem. 2009 May 1;284(18):12533-40. doi: 10.1074/jbc.M808587200. Epub 2009 Feb 26. J Biol Chem. 2009. PMID: 19251693 Free PMC article.
-
Formins: signaling effectors for assembly and polarization of actin filaments.J Cell Sci. 2003 Jul 1;116(Pt 13):2603-11. doi: 10.1242/jcs.00611. J Cell Sci. 2003. PMID: 12775772 Review.
Cited by
-
Formin 1 Regulates Ectoplasmic Specialization in the Rat Testis Through Its Actin Nucleation and Bundling Activity.Endocrinology. 2015 Aug;156(8):2969-83. doi: 10.1210/en.2015-1161. Epub 2015 Apr 22. Endocrinology. 2015. PMID: 25901598 Free PMC article.
-
Review of the mechanism of processive actin filament elongation by formins.Cell Motil Cytoskeleton. 2009 Aug;66(8):606-17. doi: 10.1002/cm.20379. Cell Motil Cytoskeleton. 2009. PMID: 19459187 Free PMC article. Review.
-
Single-molecule visualization of a formin-capping protein 'decision complex' at the actin filament barbed end.Nat Commun. 2015 Nov 13;6:8707. doi: 10.1038/ncomms9707. Nat Commun. 2015. PMID: 26566078 Free PMC article.
-
Actin filament dynamics at barbed ends: New structures, new insights.Curr Opin Cell Biol. 2024 Oct;90:102419. doi: 10.1016/j.ceb.2024.102419. Epub 2024 Aug 22. Curr Opin Cell Biol. 2024. PMID: 39178734 Free PMC article. Review.
-
Dissection of two parallel pathways for formin-mediated actin filament elongation.J Biol Chem. 2018 Nov 16;293(46):17917-17928. doi: 10.1074/jbc.RA118.004845. Epub 2018 Sep 28. J Biol Chem. 2018. PMID: 30266808 Free PMC article.
References
-
- Sagot I, Klee SK, Pellman D. Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat Cell Biol. 2002;4:42–50. - PubMed
-
- Feierbach B, Chang F. Roles of the fission yeast formin for3p in cell polarity, actin cable formation and symmetric cell division. Curr Biol. 2001;11:1656–1665. - PubMed
-
- Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol. 1999;1:136–143. - PubMed
-
- Pellegrin S, Mellor H. The Rho family GTPase Rif induces filopodia through mDia2. Curr Biol. 2005;15:129–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

