Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling
- PMID: 18157127
- PMCID: PMC2397541
- DOI: 10.1038/ng.2007.80
Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling
Abstract
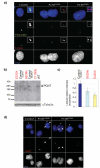
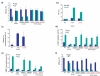
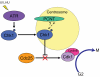
Large brain size is one of the defining characteristics of modern humans. Seckel syndrome (MIM 210600), a disorder of markedly reduced brain and body size, is associated with defective ATR-dependent DNA damage signaling. Only a single hypomorphic mutation of ATR has been identified in this genetically heterogeneous condition. We now report that mutations in the gene encoding pericentrin (PCNT)--resulting in the loss of pericentrin from the centrosome, where it has key functions anchoring both structural and regulatory proteins--also cause Seckel syndrome. Furthermore, we find that cells of individuals with Seckel syndrome due to mutations in PCNT (PCNT-Seckel) have defects in ATR-dependent checkpoint signaling, providing the first evidence linking a structural centrosomal protein with DNA damage signaling. These findings also suggest that other known microcephaly genes implicated in either DNA repair responses or centrosomal function may act in common developmental pathways determining human brain and body size.
Figures

Similar articles
-
CtIP Mutations Cause Seckel and Jawad Syndromes.PLoS Genet. 2011 Oct;7(10):e1002310. doi: 10.1371/journal.pgen.1002310. Epub 2011 Oct 6. PLoS Genet. 2011. PMID: 21998596 Free PMC article.
-
Identification of the first ATRIP-deficient patient and novel mutations in ATR define a clinical spectrum for ATR-ATRIP Seckel Syndrome.PLoS Genet. 2012;8(11):e1002945. doi: 10.1371/journal.pgen.1002945. Epub 2012 Nov 8. PLoS Genet. 2012. PMID: 23144622 Free PMC article.
-
Molecular analysis of pericentrin gene (PCNT) in a series of 24 Seckel/microcephalic osteodysplastic primordial dwarfism type II (MOPD II) families.J Med Genet. 2010 Dec;47(12):797-802. doi: 10.1136/jmg.2009.067298. Epub 2009 Jul 29. J Med Genet. 2010. PMID: 19643772
-
An overview of three new disorders associated with genetic instability: LIG4 syndrome, RS-SCID and ATR-Seckel syndrome.DNA Repair (Amst). 2004 Aug-Sep;3(8-9):1227-35. doi: 10.1016/j.dnarep.2004.03.025. DNA Repair (Amst). 2004. PMID: 15279811 Review.
-
Microcephalin: a causal link between impaired damage response signalling and microcephaly.Cell Cycle. 2006 Oct;5(20):2339-44. doi: 10.4161/cc.5.20.3358. Epub 2006 Oct 16. Cell Cycle. 2006. PMID: 17102619 Review.
Cited by
-
Opposing effects of pericentrin and microcephalin on the pericentriolar material regulate CHK1 activation in the DNA damage response.Oncogene. 2016 Apr 14;35(15):2003-10. doi: 10.1038/onc.2015.257. Epub 2015 Jul 13. Oncogene. 2016. PMID: 26165835
-
Speriolin is a novel human and mouse sperm centrosome protein.Hum Reprod. 2010 Aug;25(8):1884-94. doi: 10.1093/humrep/deq138. Epub 2010 Jun 11. Hum Reprod. 2010. PMID: 20542897 Free PMC article.
-
Pericentrin in cellular function and disease.J Cell Biol. 2010 Jan 25;188(2):181-90. doi: 10.1083/jcb.200908114. Epub 2009 Dec 1. J Cell Biol. 2010. PMID: 19951897 Free PMC article. Review.
-
FancJ regulates interstrand crosslinker induced centrosome amplification through the activation of polo-like kinase 1.Biol Open. 2013 Aug 6;2(10):1022-31. doi: 10.1242/bio.20135801. eCollection 2013. Biol Open. 2013. PMID: 24167712 Free PMC article.
-
Genetic defects in human pericentrin are associated with severe insulin resistance and diabetes.Diabetes. 2011 Mar;60(3):925-35. doi: 10.2337/db10-1334. Epub 2011 Jan 26. Diabetes. 2011. PMID: 21270239 Free PMC article.
References
-
- Majewski F, et al. Studies of microcephalic primordial dwarfism I: approach to a delineation of the Seckel syndrome. Am J Med Genet. 1982;12:7–21. - PubMed
-
- Seckel HPG. Bird-headed Dwarfs: Studies in Developmental Anthropology Including Human Proportions. Springfield, Illinois: Charles C Thomas; 1960.
-
- Alderton GK, et al. Seckel syndrome exhibits cellular features demonstrating defects in the ATR-signalling pathway. Hum Mol Genet. 2004;13:3127–38. - PubMed
-
- O'Driscoll M, et al. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet. 2003;33:497–501. - PubMed
-
- Diviani D, et al. Pericentrin anchors protein kinase A at the centrosome through a newly identified RII-binding domain. Curr Biol. 2000;10:417–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

