Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer
- PMID: 18156212
- PMCID: PMC2189611
- DOI: 10.2353/ajpath.2008.070602
Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer
Abstract
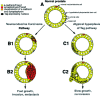
The transgenic adenocarcinoma of mouse prostate (TRAMP) model is widely used in prostate cancer research because of rapid tumor onset and progression. The transgenic mouse is on a C57BL/6 (B6) background and expresses SV40 T-antigen under the probasin promoter. The strong genetic component of susceptibility to prostate cancer in humans prompted us to investigate the effect of mouse strain background (FVB and B6) on incidence, progression, and pathology of prostate cancer in this model. Because TRAMP lesions are unique but differ from conventional prostatic intraepithelial neoplasia because the epithelium and stroma are affected diffusely, we designated them as "atypical hyperplasia of Tag." Although the incidence and severity of atypical hyperplasia of Tag is similar, FVB-TRAMP mice live significantly shorter lives than B6-TRAMP mice because of the rapid development and progression of neuroendocrine carcinomas. This is associated with an increased frequency of neuroendocrine precursor lesions in young TRAMP mice, detectable at 4 weeks after birth. These lesions show properties of bipotential stem cells and co-express markers of epithelial (E-cadherin) and neuroendocrine (synaptophysin) lineages, as well as the transcription factors Foxa1 and Foxa2. Transplantation studies using TRAMP prostatic ducts suggested that neuroendocrine carcinomas arise independently from atypical hyperplasias or other epithelial lesions. Adenocarcinomas were not seen in our cohort. Thus, neuroendocrine carcinomas are the principal malignancy in this model and may develop from bipotential progenitor cells at an early stage of prostate tumorigenesis.
Figures



Similar articles
-
A probasin-large T antigen transgenic mouse line develops prostate adenocarcinoma and neuroendocrine carcinoma with metastatic potential.Cancer Res. 2001 Mar 1;61(5):2239-49. Cancer Res. 2001. PMID: 11280793
-
Lobe-specific lineages of carcinogenesis in the transgenic adenocarcinoma of mouse prostate and their responses to chemopreventive selenium.Prostate. 2011 Sep 15;71(13):1429-40. doi: 10.1002/pros.21360. Epub 2011 Feb 25. Prostate. 2011. PMID: 21360561
-
Extra-prostatic transgene-associated neoplastic lesions in transgenic adenocarcinoma of the mouse prostate (TRAMP) mice.Toxicol Pathol. 2015 Feb;43(2):186-97. doi: 10.1177/0192623314531351. Epub 2014 Apr 17. Toxicol Pathol. 2015. PMID: 24742627 Free PMC article.
-
Genetically modified mice and their use in developing therapeutic strategies for prostate cancer.J Urol. 2004 Jul;172(1):12-9. doi: 10.1097/01.ju.0000132122.93436.aa. J Urol. 2004. PMID: 15201729 Review.
-
Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) model: A good alternative to study PCa progression and chemoprevention approaches.Life Sci. 2019 Jan 15;217:141-147. doi: 10.1016/j.lfs.2018.12.002. Epub 2018 Dec 4. Life Sci. 2019. PMID: 30528182 Review.
Cited by
-
Emerging Perspectives in Zinc Transporter Research in Prostate Cancer: An Updated Review.Nutrients. 2024 Jun 26;16(13):2026. doi: 10.3390/nu16132026. Nutrients. 2024. PMID: 38999774 Free PMC article. Review.
-
2,3,7,8-Tetrachlorodibenzo-p-dioxin has both pro-carcinogenic and anti-carcinogenic effects on neuroendocrine prostate carcinoma formation in TRAMP mice.Toxicol Appl Pharmacol. 2016 Aug 15;305:242-249. doi: 10.1016/j.taap.2016.04.018. Epub 2016 May 3. Toxicol Appl Pharmacol. 2016. PMID: 27151233 Free PMC article.
-
In vitro and in vivo model systems used in prostate cancer research.J Biol Methods. 2015;2(1):e17. doi: 10.14440/jbm.2015.63. J Biol Methods. 2015. PMID: 26146646 Free PMC article.
-
ADORA2A-driven proline synthesis triggers epigenetic reprogramming in neuroendocrine prostate and lung cancers.J Clin Invest. 2023 Dec 15;133(24):e168670. doi: 10.1172/JCI168670. J Clin Invest. 2023. PMID: 38099497 Free PMC article.
-
Suppression of prostate epithelial proliferation and intraprostatic progrowth signaling in transgenic mice by a new energy restriction-mimetic agent.Cancer Prev Res (Phila). 2013 Mar;6(3):232-41. doi: 10.1158/1940-6207.CAPR-12-0057. Epub 2012 Dec 28. Cancer Prev Res (Phila). 2013. PMID: 23275006 Free PMC article.
References
-
- Chan JM, Jou RM, Carroll PR. The relative impact and future burden of prostate cancer in the United States. J Urol. 2004;172:S13–S17. - PubMed
-
- Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. - PubMed
-
- Abate-Shen C, Shen MM. Mouse models of prostate carcinogenesis. Trends Genet. 2002;18:S1–S5. - PubMed
-
- Navone NM, Logothetis CJ, von Eschenbach AC, Troncoso P. Model systems of prostate cancer: uses and limitations. Cancer Metastasis Rev. 1998;17:361–371. - PubMed
-
- Powell WC, Cardiff RD, Cohen MB, Miller GJ, Roy-Burman P. Mouse strains for prostate tumorigenesis based on genes altered in human prostate cancer. Curr Drug Targets. 2003;4:263–279. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

