An essential role for fibronectin extra type III domain A in pulmonary fibrosis
- PMID: 18096707
- PMCID: PMC2267338
- DOI: 10.1164/rccm.200708-1291OC
An essential role for fibronectin extra type III domain A in pulmonary fibrosis
Abstract
Rationale: Tissue fibrosis is considered a dysregulated wound-healing response. Fibronectin containing extra type III domain A (EDA) is implicated in the regulation of wound healing. EDA-containing fibronectin is deposited during wound repair, and its presence precedes that of collagen.
Objectives: To investigate the role of EDA-containing fibronectin in lung fibrogenesis.
Methods: Primary lung fibroblasts from patients with idiopathic pulmonary fibrosis or from patients undergoing resection for lung cancer were assessed for EDA-containing fibronectin and alpha-smooth muscle actin (alpha-SMA) expression. Mice lacking the EDA domain of fibronectin and their wild-type littermates were challenged with the bleomycin model of lung fibrosis. Primary lung fibroblasts from these mice were assayed in vitro to determine the contribution of EDA-containing fibronectin to fibroblast phenotypes.
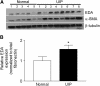
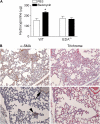
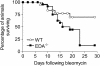
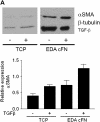
Measurements and main results: Idiopathic pulmonary fibrosis lung fibroblasts produced markedly more EDA-containing fibronectin and alpha-SMA than control fibroblasts. EDA-null mice failed to develop significant fibrosis 21 days after bleomycin challenge, whereas wild-type controls developed the expected increase in total lung collagen. Histologic analysis of EDA-null lungs after bleomycin showed less collagen and fewer alpha-SMA-expressing myofibroblasts compared with that observed in wild-type mice. Failure to develop lung fibrosis in EDA-null mice correlated with diminished activation of latent transforming growth factor (TGF)-beta and decreased lung fibroblast responsiveness to active TGF-beta in vitro.
Conclusions: The data show that EDA-containing fibronectin is essential for the fibrotic resolution of lung injury through TGF-beta activation and responsiveness, and suggest that EDA-containing fibronectin plays a critical role in tissue fibrogenesis.
Figures




Similar articles
-
Lysophosphatidic acid receptor-2 deficiency confers protection against bleomycin-induced lung injury and fibrosis in mice.Am J Respir Cell Mol Biol. 2013 Dec;49(6):912-22. doi: 10.1165/rcmb.2013-0070OC. Am J Respir Cell Mol Biol. 2013. PMID: 23808384 Free PMC article.
-
In vitro cultured fetal fibroblasts have myofibroblast-associated characteristics and produce a fibrotic-like environment upon stimulation with TGF-β1: Is there a thin line between fetal scarless healing and fibrosis?Arch Dermatol Res. 2017 Mar;309(2):111-121. doi: 10.1007/s00403-016-1710-3. Epub 2016 Dec 21. Arch Dermatol Res. 2017. PMID: 28004279
-
Therapeutic effect of a peptide inhibitor of TGF-β on pulmonary fibrosis.Cytokine. 2011 Mar;53(3):327-33. doi: 10.1016/j.cyto.2010.11.019. Epub 2010 Dec 23. Cytokine. 2011. PMID: 21185199
-
EDA-containing cellular fibronectin induces fibroblast differentiation through binding to alpha4beta7 integrin receptor and MAPK/Erk 1/2-dependent signaling.FASEB J. 2010 Nov;24(11):4503-12. doi: 10.1096/fj.10-154435. Epub 2010 Jul 19. FASEB J. 2010. PMID: 20643910 Free PMC article.
-
Emerging concepts in the pathogenesis of lung fibrosis.Am J Pathol. 2009 Jul;175(1):3-16. doi: 10.2353/ajpath.2009.081170. Epub 2009 Jun 4. Am J Pathol. 2009. PMID: 19497999 Free PMC article. Review.
Cited by
-
MicroRNA-29b inhibits TGF-β1-induced fibrosis via regulation of the TGF-β1/Smad pathway in primary human endometrial stromal cells.Mol Med Rep. 2016 May;13(5):4229-37. doi: 10.3892/mmr.2016.5062. Epub 2016 Mar 30. Mol Med Rep. 2016. PMID: 27035110 Free PMC article.
-
Extracellular matrix dynamics and fetal membrane rupture.Reprod Sci. 2013 Feb;20(2):140-53. doi: 10.1177/1933719111424454. Epub 2012 Jan 19. Reprod Sci. 2013. PMID: 22267536 Free PMC article. Review.
-
Recent developments in myofibroblast biology: paradigms for connective tissue remodeling.Am J Pathol. 2012 Apr;180(4):1340-55. doi: 10.1016/j.ajpath.2012.02.004. Epub 2012 Mar 2. Am J Pathol. 2012. PMID: 22387320 Free PMC article. Review.
-
Cytokine priming enhances the antifibrotic effects of human adipose derived mesenchymal stromal cells conditioned medium.Stem Cell Res Ther. 2024 Sep 27;15(1):329. doi: 10.1186/s13287-024-03916-9. Stem Cell Res Ther. 2024. PMID: 39334258 Free PMC article.
-
Substance P induces fibrotic changes through activation of the RhoA/ROCK pathway in an in vitro human corneal fibrosis model.J Mol Med (Berl). 2019 Oct;97(10):1477-1489. doi: 10.1007/s00109-019-01827-4. Epub 2019 Aug 9. J Mol Med (Berl). 2019. PMID: 31399750 Free PMC article.
References
-
- Gharaee-Kermani M, Gyetko MR, Hu B, Phan SH. New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis: a potential role for stem cells in the lung parenchyma and implications for therapy. Pharm Res 2007;24:819–841. - PubMed
-
- Hernnas J, Nettelbladt O, Bjermer L, Sarnstrand B, Malmstrom A, Hallgren R. Alveolar accumulation of fibronectin and hyaluronan precedes bleomycin-induced pulmonary fibrosis in the rat. Eur Respir J 1992;5:404–410. - PubMed
-
- Kuhn C III, Boldt J, King TE Jr, Crouch E, Vartio T, McDonald JA. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis 1989;140:1693–1703. - PubMed
-
- Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci 2002;115:3861–3863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

