Thiacetazone, an antitubercular drug that inhibits cyclopropanation of cell wall mycolic acids in mycobacteria
- PMID: 18094751
- PMCID: PMC2147073
- DOI: 10.1371/journal.pone.0001343
Thiacetazone, an antitubercular drug that inhibits cyclopropanation of cell wall mycolic acids in mycobacteria
Abstract
Background: Mycolic acids are a complex mixture of branched, long-chain fatty acids, representing key components of the highly hydrophobic mycobacterial cell wall. Pathogenic mycobacteria carry mycolic acid sub-types that contain cyclopropane rings. Double bonds at specific sites on mycolic acid precursors are modified by the action of cyclopropane mycolic acid synthases (CMASs). The latter belong to a family of S-adenosyl-methionine-dependent methyl transferases, of which several have been well studied in Mycobacterium tuberculosis, namely, MmaA1 through A4, PcaA and CmaA2. Cyclopropanated mycolic acids are key factors participating in cell envelope permeability, host immunomodulation and persistence of M. tuberculosis. While several antitubercular agents inhibit mycolic acid synthesis, to date, the CMASs have not been shown to be drug targets.
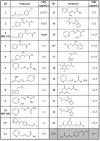
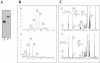
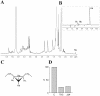
Methodology/principle findings: We have employed various complementary approaches to show that the antitubercular drug, thiacetazone (TAC), and its chemical analogues, inhibit mycolic acid cyclopropanation. Dramatic changes in the content and ratio of mycolic acids in the vaccine strain Mycobacterium bovis BCG, as well as in the related pathogenic species Mycobacterium marinum were observed after treatment with the drugs. Combination of thin layer chromatography, mass spectrometry and Nuclear Magnetic Resonance (NMR) analyses of mycolic acids purified from drug-treated mycobacteria showed a significant loss of cyclopropanation in both the alpha- and oxygenated mycolate sub-types. Additionally, High-Resolution Magic Angle Spinning (HR-MAS) NMR analyses on whole cells was used to detect cell wall-associated mycolates and to quantify the cyclopropanation status of the cell envelope. Further, overexpression of cmaA2, mmaA2 or pcaA in mycobacteria partially reversed the effects of TAC and its analogue on mycolic acid cyclopropanation, suggesting that the drugs act directly on CMASs.
Conclusions/significance: This is a first report on the mechanism of action of TAC, demonstrating the CMASs as its cellular targets in mycobacteria. The implications of this study may be important for the design of alternative strategies for tuberculosis treatment.
Conflict of interest statement
Figures



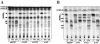
Similar articles
-
Temperature-dependent regulation of mycolic acid cyclopropanation in saprophytic mycobacteria: role of the Mycobacterium smegmatis 1351 gene (MSMEG_1351) in CIS-cyclopropanation of alpha-mycolates.J Biol Chem. 2010 Jul 9;285(28):21698-707. doi: 10.1074/jbc.M110.125724. Epub 2010 May 10. J Biol Chem. 2010. PMID: 20457615 Free PMC article.
-
Redundant function of cmaA2 and mmaA2 in Mycobacterium tuberculosis cis cyclopropanation of oxygenated mycolates.J Bacteriol. 2010 Jul;192(14):3661-8. doi: 10.1128/JB.00312-10. Epub 2010 May 14. J Bacteriol. 2010. PMID: 20472794 Free PMC article.
-
Mycolic acid methyltransferase, MmaA4, is necessary for thiacetazone susceptibility in Mycobacterium tuberculosis.Mol Microbiol. 2009 Mar;71(5):1263-77. doi: 10.1111/j.1365-2958.2009.06604.x. Epub 2009 Jan 15. Mol Microbiol. 2009. PMID: 19183278
-
[Mycolic acids--biological role and potential application in Mycobacterium detection and differentiation].Postepy Hig Med Dosw (Online). 2014 Apr 4;68:350-8. doi: 10.5604/17322693.1097425. Postepy Hig Med Dosw (Online). 2014. PMID: 24864086 Review. Polish.
-
Drugs that inhibit mycolic acid biosynthesis in Mycobacterium tuberculosis.Curr Pharm Biotechnol. 2002 Sep;3(3):197-225. doi: 10.2174/1389201023378328. Curr Pharm Biotechnol. 2002. PMID: 12164478 Review.
Cited by
-
Xanthates: Metabolism by Flavoprotein-Containing Monooxygenases and Antimycobacterial Activity.Drug Metab Dispos. 2018 Aug;46(8):1091-1095. doi: 10.1124/dmd.118.081984. Epub 2018 May 18. Drug Metab Dispos. 2018. PMID: 29777023 Free PMC article.
-
Phenylethyl butyrate enhances the potency of second-line drugs against clinical isolates of Mycobacterium tuberculosis.Antimicrob Agents Chemother. 2012 Feb;56(2):1142-5. doi: 10.1128/AAC.05649-11. Epub 2011 Nov 21. Antimicrob Agents Chemother. 2012. PMID: 22106218 Free PMC article.
-
Molecule Property Analyses of Active Compounds for Mycobacterium tuberculosis.J Med Chem. 2020 Sep 10;63(17):8917-8955. doi: 10.1021/acs.jmedchem.9b02075. Epub 2020 Apr 20. J Med Chem. 2020. PMID: 32259446 Free PMC article. Review.
-
Resistance to Thiacetazone Derivatives Active against Mycobacterium abscessus Involves Mutations in the MmpL5 Transcriptional Repressor MAB_4384.Antimicrob Agents Chemother. 2017 Mar 24;61(4):e02509-16. doi: 10.1128/AAC.02509-16. Print 2017 Apr. Antimicrob Agents Chemother. 2017. PMID: 28096157 Free PMC article.
-
A Coumarin-Based Analogue of Thiacetazone as Dual Covalent Inhibitor and Potential Fluorescent Label of HadA in Mycobacterium tuberculosis.ACS Infect Dis. 2021 Mar 12;7(3):552-565. doi: 10.1021/acsinfecdis.0c00325. Epub 2021 Feb 22. ACS Infect Dis. 2021. PMID: 33617235 Free PMC article.
References
-
- Dorman SE, Chaisson RE. From magic bullets back to the magic mountain: the rise of extensively drug-resistant tuberculosis. Nat Med. 2007;13:295–298. - PubMed
-
- Raviglione MC, Smith IM. XDR tuberculosis–implications for global public health. N Engl J Med. 2007;356:656–659. - PubMed
-
- Prevention CfDCa. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs–worldwide, 2000–2004. MMWR Morb Mortal Wkly Rep. 2006;55:301–305. - PubMed
-
- Davidson PT, Le HQ. Drug treatment of tuberculosis–1992. Drugs. 1992;43:651–673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

