The GSK3 hypothesis of Alzheimer's disease
- PMID: 18088381
- PMCID: PMC3073119
- DOI: 10.1111/j.1471-4159.2007.05194.x
The GSK3 hypothesis of Alzheimer's disease
Abstract
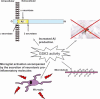
Glycogen synthase kinase 3 (GSK3) is a constitutively active, proline-directed serine/threonine kinase that plays a part in a number of physiological processes ranging from glycogen metabolism to gene transcription. GSK3 also plays a pivotal and central role in the pathogenesis of both sporadic and familial forms of Alzheimer's disease (AD), an observation that has led us to coin the 'GSK3 hypothesis of AD'. According to this hypothesis, over-activity of GSK3 accounts for memory impairment, tau hyper-phosphorylation, increased beta-amyloid production and local plaque-associated microglial-mediated inflammatory responses; all of which are hallmark characteristics of AD. If our 'GSK3 hypothesis of AD' is substantiated and GSK3 is indeed a causal mediator of AD then inhibitors of GSK3 would provide a novel avenue for therapeutic intervention in this devastating disorder.
Figures
Similar articles
-
GSK3 in Alzheimer's disease: mind the isoforms.J Alzheimers Dis. 2014;39(4):707-10. doi: 10.3233/JAD-131661. J Alzheimers Dis. 2014. PMID: 24254703 Free PMC article. Review.
-
GSK3 and tau: two convergence points in Alzheimer's disease.J Alzheimers Dis. 2013;33 Suppl 1:S141-4. doi: 10.3233/JAD-2012-129025. J Alzheimers Dis. 2013. PMID: 22710914 Review.
-
Therapeutic implications of glycogen synthase kinase-3β in Alzheimer's disease: a novel therapeutic target.Int J Neurosci. 2024 Jun;134(6):603-619. doi: 10.1080/00207454.2022.2130297. Epub 2022 Oct 10. Int J Neurosci. 2024. PMID: 36178363 Review.
-
Amyloid beta-induced glycogen synthase kinase 3β phosphorylated VDAC1 in Alzheimer's disease: implications for synaptic dysfunction and neuronal damage.Biochim Biophys Acta. 2013 Dec;1832(12):1913-21. doi: 10.1016/j.bbadis.2013.06.012. Epub 2013 Jun 28. Biochim Biophys Acta. 2013. PMID: 23816568 Free PMC article. Review.
-
Glycogen Synthase Kinase 3 (GSK3): Its Role and Inhibitors.Curr Top Med Chem. 2020;20(17):1522-1534. doi: 10.2174/1568026620666200516153136. Curr Top Med Chem. 2020. PMID: 32416693 Review.
Cited by
-
GSK3beta-mediated Drp1 phosphorylation induced elongated mitochondrial morphology against oxidative stress.PLoS One. 2012;7(11):e49112. doi: 10.1371/journal.pone.0049112. Epub 2012 Nov 20. PLoS One. 2012. PMID: 23185298 Free PMC article.
-
N-butylidenephthalide attenuates Alzheimer's disease-like cytopathy in Down syndrome induced pluripotent stem cell-derived neurons.Sci Rep. 2015 Mar 4;5:8744. doi: 10.1038/srep08744. Sci Rep. 2015. PMID: 25735452 Free PMC article.
-
The Oxindole Derivatives, New Promising GSK-3β Inhibitors as One of the Potential Treatments for Alzheimer's Disease-A Molecular Dynamics Approach.Biology (Basel). 2021 Apr 15;10(4):332. doi: 10.3390/biology10040332. Biology (Basel). 2021. PMID: 33920768 Free PMC article.
-
A new look at an old drug: neuroprotective effects and therapeutic potentials of lithium salts.Neuropsychiatr Dis Treat. 2016 Jul 11;12:1687-703. doi: 10.2147/NDT.S106479. eCollection 2016. Neuropsychiatr Dis Treat. 2016. PMID: 27468233 Free PMC article. Review.
-
Tau Exon 10 Inclusion by PrPC through Downregulating GSK3β Activity.Int J Mol Sci. 2021 May 20;22(10):5370. doi: 10.3390/ijms22105370. Int J Mol Sci. 2021. PMID: 34065232 Free PMC article.
References
-
- Alvarez G, Munoz-Montano JR, Satrustegui J, Avila J, Bogonez E, az-Nido J. Lithium protects cultured neurons against beta-amyloid-induced neurodegeneration. FEBS Lett. 1999;453:260–264. - PubMed
-
- Anderton BH, Betts J, Blackstock WP, et al. Sites of phosphorylation in tau and factors affecting their regulation. Biochem. Soc. Symp. 2001;67:73–80. - PubMed
-
- Asuni AA, Hooper C, Reynolds CH, Lovestone S, Anderton BH, Killick R. GSK3alpha exhibits beta-catenin and tau directed kinase activities that are modulated by Wnt. Eur. J. Neurosci. 2006;24:3387–3392. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

