ARF1 is directly involved in dynamin-independent endocytosis
- PMID: 18084285
- PMCID: PMC7617176
- DOI: 10.1038/ncb1666
ARF1 is directly involved in dynamin-independent endocytosis
Abstract
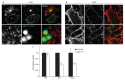
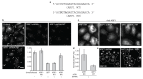
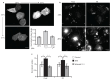
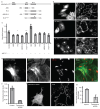
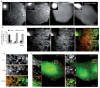
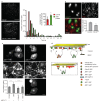
Endocytosis of glycosylphosphatidyl inositol (GPI)-anchored proteins (GPI-APs) and the fluid phase takes place primarily through a dynamin- and clathrin-independent, Cdc42-regulated pinocytic mechanism. This mechanism is mediated by primary carriers called clathrin-independent carriers (CLICs), which fuse to form tubular early endocytic compartments called GPI-AP enriched endosomal compartments (GEECs). Here, we show that reduction in activity or levels of ARF1 specifically inhibits GPI-AP and fluid-phase endocytosis without affecting other clathrin-dependent or independent endocytic pathways. ARF1 is activated at distinct sites on the plasma membrane, and by the recruitment of RhoGAP domain-containing protein, ARHGAP10, to the plasma membrane, modulates cell-surface Cdc42 dynamics. This results in the coupling of ARF1 and Cdc42 activity to regulate endocytosis at the plasma membrane. These findings provide a molecular basis for a crosstalk of endocytosis with secretion by the sharing of a key regulator of secretory traffic, ARF1.
Figures

Similar articles
-
Cholesterol-sensitive Cdc42 activation regulates actin polymerization for endocytosis via the GEEC pathway.Traffic. 2007 Jun;8(6):702-17. doi: 10.1111/j.1600-0854.2007.00565.x. Epub 2007 Apr 25. Traffic. 2007. PMID: 17461795 Free PMC article.
-
Enabling Systemic Identification and Functionality Profiling for Cdc42 Homeostatic Modulators.bioRxiv [Preprint]. 2024 Jan 8:2024.01.05.574351. doi: 10.1101/2024.01.05.574351. bioRxiv. 2024. Update in: Commun Chem. 2024 Nov 19;7(1):271. doi: 10.1038/s42004-024-01352-7 PMID: 38260445 Free PMC article. Updated. Preprint.
-
Clathrin-independent pathways of endocytosis.Cold Spring Harb Perspect Biol. 2014 Jun 2;6(6):a016758. doi: 10.1101/cshperspect.a016758. Cold Spring Harb Perspect Biol. 2014. PMID: 24890511 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article. Review.
Cited by
-
Arf6 promotes autophagosome formation via effects on phosphatidylinositol 4,5-bisphosphate and phospholipase D.J Cell Biol. 2012 Feb 20;196(4):483-96. doi: 10.1083/jcb.201110114. J Cell Biol. 2012. PMID: 22351926 Free PMC article.
-
Brefeldin A-inhibited ADP-ribosylation factor activator BIG2 regulates cell migration via integrin β1 cycling and actin remodeling.Proc Natl Acad Sci U S A. 2012 Sep 4;109(36):14464-9. doi: 10.1073/pnas.1211877109. Epub 2012 Aug 20. Proc Natl Acad Sci U S A. 2012. PMID: 22908276 Free PMC article.
-
Endocytosis of gene delivery vectors: from clathrin-dependent to lipid raft-mediated endocytosis.Mol Ther. 2013 Jun;21(6):1118-30. doi: 10.1038/mt.2013.54. Epub 2013 Apr 16. Mol Ther. 2013. PMID: 23587924 Free PMC article. Review.
-
Transport of influenza virus neuraminidase (NA) to host cell surface is regulated by ARHGAP21 and Cdc42 proteins.J Biol Chem. 2012 Mar 23;287(13):9804-9816. doi: 10.1074/jbc.M111.312959. Epub 2012 Feb 8. J Biol Chem. 2012. PMID: 22318733 Free PMC article.
-
Salt-induced remodeling of spatially restricted clathrin-independent endocytic pathways in Arabidopsis root.Plant Cell. 2015 Apr;27(4):1297-315. doi: 10.1105/tpc.15.00154. Epub 2015 Apr 21. Plant Cell. 2015. PMID: 25901088 Free PMC article.
References
-
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. - PubMed
-
- Kirkham M, Parton RG. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta. 2005;1746:350–363. - PubMed
-
- Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2:411–423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

