TRAIL-R deficiency in mice enhances lymph node metastasis without affecting primary tumor development
- PMID: 18079967
- PMCID: PMC2129237
- DOI: 10.1172/JCI33061
TRAIL-R deficiency in mice enhances lymph node metastasis without affecting primary tumor development
Abstract
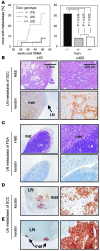
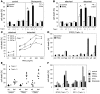
TRAIL is a promising anticancer agent due to its ability to selectively induce apoptosis in established tumor cell lines but not nontransformed cells. Herein, we demonstrate a role for the apoptosis-inducing TRAIL receptor (TRAIL-R) as a metastasis suppressor. Although mouse models employing tumor transplantation have shown that TRAIL can reduce tumor growth, autochthonous tumor models have generated conflicting results with respect to the physiological role of the TRAIL system during tumorigenesis. We used a multistage model of squamous cell carcinoma to examine the role of TRAIL-R throughout all steps of tumor development. DMBA/TPA-treated TRAIL-R-deficient mice showed neither an increase in number or growth rate of benign papillomas nor an increase in the rate of progression to squamous cell carcinoma. However, metastasis to lymph nodes was significantly enhanced, indicating a role for TRAIL-R specifically in the suppression of metastasis. We also found that adherent TRAIL-R-expressing skin carcinoma cells were TRAIL resistant in vitro but were sensitized to TRAIL upon detachment by inactivation of the ERK signaling pathway. As detachment from the primary tumor is an obligatory step in metastasis, this provides a possible mechanism by which TRAIL-R could inhibit metastasis. Hence, treatment of cancer patients with agonists of the apoptosis-inducing receptors for TRAIL may prove useful in reducing the incidence of metastasis.
Figures



Comment in
-
TRAIL-R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis.J Clin Invest. 2008 Jan;118(1):111-23. doi: 10.1172/JCI29900. J Clin Invest. 2008. PMID: 18079962 Free PMC article.
Similar articles
-
Prospective antitumor effects of the combination of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and cisplatin against esophageal squamous cell carcinoma.Surg Today. 2006;36(11):966-74. doi: 10.1007/s00595-006-3295-5. Surg Today. 2006. PMID: 17072716
-
Metastasis suppressor function of tumor necrosis factor-related apoptosis-inducing ligand-R in mice: implications for TRAIL-based therapy in humans?Cancer Res. 2008 Aug 1;68(15):6035-7. doi: 10.1158/0008-5472.CAN-08-0078. Cancer Res. 2008. PMID: 18676822 Free PMC article. Review.
-
The long non-coding RNA HOTAIR enhances pancreatic cancer resistance to TNF-related apoptosis-inducing ligand.J Biol Chem. 2017 Jun 23;292(25):10390-10397. doi: 10.1074/jbc.M117.786830. Epub 2017 May 5. J Biol Chem. 2017. PMID: 28476883 Free PMC article.
-
Apigenin induces apoptosis via tumor necrosis factor receptor- and Bcl-2-mediated pathway and enhances susceptibility of head and neck squamous cell carcinoma to 5-fluorouracil and cisplatin.Biochim Biophys Acta. 2012 Jul;1820(7):1081-91. doi: 10.1016/j.bbagen.2012.04.013. Epub 2012 Apr 24. Biochim Biophys Acta. 2012. PMID: 22554915
-
Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy.Exp Biol Med (Maywood). 2015 Jun;240(6):760-73. doi: 10.1177/1535370215579167. Epub 2015 Apr 7. Exp Biol Med (Maywood). 2015. PMID: 25854879 Free PMC article. Review.
Cited by
-
Th-MYCN mice with caspase-8 deficiency develop advanced neuroblastoma with bone marrow metastasis.Cancer Res. 2013 Jul 1;73(13):4086-97. doi: 10.1158/0008-5472.CAN-12-2681. Epub 2013 Mar 27. Cancer Res. 2013. PMID: 23536557 Free PMC article.
-
H3K9 Trimethylation Silences Fas Expression To Confer Colon Carcinoma Immune Escape and 5-Fluorouracil Chemoresistance.J Immunol. 2015 Aug 15;195(4):1868-82. doi: 10.4049/jimmunol.1402243. Epub 2015 Jul 1. J Immunol. 2015. PMID: 26136424 Free PMC article.
-
The Increase in the Drug Resistance of Acute Myeloid Leukemia THP-1 Cells in High-Density Cell Culture Is Associated with Inflammatory-like Activation and Anti-Apoptotic Bcl-2 Proteins.Int J Mol Sci. 2022 Jul 17;23(14):7881. doi: 10.3390/ijms23147881. Int J Mol Sci. 2022. PMID: 35887226 Free PMC article.
-
Regulating TRAIL receptor-induced cell death at the membrane : a deadly discussion.Recent Pat Anticancer Drug Discov. 2011 Sep;6(3):311-23. doi: 10.2174/157489211796957757. Recent Pat Anticancer Drug Discov. 2011. PMID: 21756247 Free PMC article. Review.
-
Tumor necrosis factor-related apoptosis-inducing ligand additive with Iodine-131 of inhibits non-small cell lung cancer cells through promoting apoptosis.Oncol Lett. 2018 Jul;16(1):276-284. doi: 10.3892/ol.2018.8635. Epub 2018 May 4. Oncol Lett. 2018. PMID: 29928412 Free PMC article.
References
-
- Falschlehner C., Emmerich C.H., Gerlach B., Walczak H. TRAIL signalling: Decisions between life and death. Int. J. Biochem. Cell Biol. 2007;39:1462–1475. - PubMed
-
- Wu G.S., Burns T.F., Zhan Y., Alnemri E.S., El-Deiry W.S. Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res. 1999;59:2770–2775. - PubMed
-
- Walczak H., et al. Tumoricidal activity of tumor necrosis factor-related apoptosis- inducing ligand in vivo. Nat. Med. 1999;5:157–163. - PubMed
-
- Koschny R., Walczak H., Ganten T.M. The promise of TRAIL-potential and risks of a novel anticancer therapy. J. Mol. Med. 2007;85:923–935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

