Intermediate-affinity LFA-1 binds alpha-actinin-1 to control migration at the leading edge of the T cell
- PMID: 18079697
- PMCID: PMC2147999
- DOI: 10.1038/sj.emboj.7601959
Intermediate-affinity LFA-1 binds alpha-actinin-1 to control migration at the leading edge of the T cell
Abstract
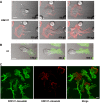
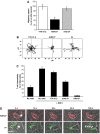
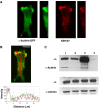
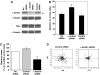
T lymphocytes use LFA-1 to migrate into lymph nodes and inflammatory sites. To investigate the mechanisms regulating this migration, we utilize mAbs selective for conformational epitopes as probes for active LFA-1. Expression of the KIM127 epitope, but not the 24 epitope, defines the extended conformation of LFA-1, which has intermediate affinity for ligand ICAM-1. A key finding is that KIM127-positive LFA-1 forms new adhesions at the T lymphocyte leading edge. This LFA-1 links to the cytoskeleton through alpha-actinin-1 and disruption at the level of integrin or actin results in loss of cell spreading and migratory speed due to a failure of attachment at the leading edge. The KIM127 pattern contrasts with high-affinity LFA-1 that expresses both 24 and KIM127 epitopes, is restricted to the mid-cell focal zone and controls ICAM-1 attachment. Identification of distinctive roles for intermediate- and high-affinity LFA-1 in T lymphocyte migration provides a biological function for two active conformations of this integrin for the first time.
Figures




Similar articles
-
CD18 activation epitopes induced by leukocyte activation.J Immunol. 2001 Dec 1;167(11):6113-22. doi: 10.4049/jimmunol.167.11.6113. J Immunol. 2001. PMID: 11714770
-
Cathepsin X cleaves the beta2 cytoplasmic tail of LFA-1 inducing the intermediate affinity form of LFA-1 and alpha-actinin-1 binding.Eur J Immunol. 2009 Nov;39(11):3217-27. doi: 10.1002/eji.200939562. Eur J Immunol. 2009. PMID: 19750481
-
LFA-1 fine-tuning by cathepsin X.IUBMB Life. 2011 Sep;63(9):686-93. doi: 10.1002/iub.505. Epub 2011 Jul 27. IUBMB Life. 2011. PMID: 21796748 Review.
-
The role of the integrin LFA-1 in T-lymphocyte migration.Immunol Rev. 2007 Aug;218:135-46. doi: 10.1111/j.1600-065X.2007.00537.x. Immunol Rev. 2007. PMID: 17624950 Review.
-
Epitope mapping of antibodies to the C-terminal region of the integrin beta 2 subunit reveals regions that become exposed upon receptor activation.J Immunol. 2001 May 1;166(9):5629-37. doi: 10.4049/jimmunol.166.9.5629. J Immunol. 2001. PMID: 11313403
Cited by
-
NFATc1 controls the cytotoxicity of CD8+ T cells.Nat Commun. 2017 Sep 11;8(1):511. doi: 10.1038/s41467-017-00612-6. Nat Commun. 2017. PMID: 28894104 Free PMC article.
-
Rolling on E- or P-selectin induces the extended but not high-affinity conformation of LFA-1 in neutrophils.Blood. 2010 Jul 29;116(4):617-24. doi: 10.1182/blood-2010-01-266122. Epub 2010 May 5. Blood. 2010. PMID: 20445017 Free PMC article.
-
STAT3-stathmin interactions control microtubule dynamics in migrating T-cells.J Biol Chem. 2009 May 1;284(18):12349-62. doi: 10.1074/jbc.M807761200. Epub 2009 Feb 26. J Biol Chem. 2009. PMID: 19251695 Free PMC article.
-
T Lymphocyte Migration: An Action Movie Starring the Actin and Associated Actors.Front Immunol. 2015 Nov 18;6:586. doi: 10.3389/fimmu.2015.00586. eCollection 2015. Front Immunol. 2015. PMID: 26635800 Free PMC article. Review.
-
Comparison of immortalized bEnd5 and primary mouse brain microvascular endothelial cells as in vitro blood-brain barrier models for the study of T cell extravasation.J Cereb Blood Flow Metab. 2011 Jan;31(1):315-27. doi: 10.1038/jcbfm.2010.96. Epub 2010 Jul 7. J Cereb Blood Flow Metab. 2011. PMID: 20606687 Free PMC article.
References
-
- Alonso JL, Essafi M, Xiong JP, Stehle T, Arnaout MA (2002) Does the integrin alphaA domain act as a ligand for its betaA domain? Curr Biol 12: R340–R342 - PubMed
-
- Beglova N, Blacklow SC, Takagi J, Springer TA (2002) Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nat Struct Biol 9: 282–287 - PubMed
-
- Carman CV, Springer TA (2003) Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol 15: 547–556 - PubMed
-
- Cyster JG (2005) Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol 23: 127–159 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

