The extracellular matrix protein TGFBI induces microtubule stabilization and sensitizes ovarian cancers to paclitaxel
- PMID: 18068629
- PMCID: PMC2148463
- DOI: 10.1016/j.ccr.2007.11.014
The extracellular matrix protein TGFBI induces microtubule stabilization and sensitizes ovarian cancers to paclitaxel
Abstract
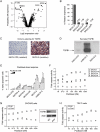
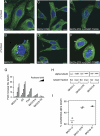
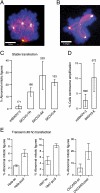
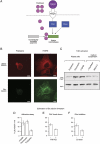
The extracellular matrix (ECM) can induce chemotherapy resistance via AKT-mediated inhibition of apoptosis. Here, we show that loss of the ECM protein TGFBI (transforming growth factor beta induced) is sufficient to induce specific resistance to paclitaxel and mitotic spindle abnormalities in ovarian cancer cells. Paclitaxel-resistant cells treated with recombinant TGFBI protein show integrin-dependent restoration of paclitaxel sensitivity via FAK- and Rho-dependent stabilization of microtubules. Immunohistochemical staining for TGFBI in paclitaxel-treated ovarian cancers from a prospective clinical trial showed that morphological changes of paclitaxel-induced cytotoxicity were restricted to areas of strong expression of TGFBI. These data show that ECM can mediate taxane sensitivity by modulating microtubule stability.
Figures

Similar articles
-
ß3 integrin modulates transforming growth factor beta induced (TGFBI) function and paclitaxel response in ovarian cancer cells.Mol Cancer. 2012 May 28;11:36. doi: 10.1186/1476-4598-11-36. Mol Cancer. 2012. PMID: 22640878 Free PMC article.
-
SPARC Regulates Transforming Growth Factor Beta Induced (TGFBI) Extracellular Matrix Deposition and Paclitaxel Response in Ovarian Cancer Cells.PLoS One. 2016 Sep 13;11(9):e0162698. doi: 10.1371/journal.pone.0162698. eCollection 2016. PLoS One. 2016. PMID: 27622658 Free PMC article.
-
SRC tyrosine kinase and multidrug resistance protein-1 inhibitions act independently but cooperatively to restore paclitaxel sensitivity to paclitaxel-resistant ovarian cancer cells.Cancer Res. 2005 Nov 15;65(22):10381-8. doi: 10.1158/0008-5472.CAN-05-1822. Cancer Res. 2005. PMID: 16288028
-
Transforming growth Factor-Beta-Induced Protein (TGFBI)/(βig-H3): a matrix protein with dual functions in ovarian cancer.Int J Mol Sci. 2012;13(8):10461-10477. doi: 10.3390/ijms130810461. Epub 2012 Aug 21. Int J Mol Sci. 2012. PMID: 22949874 Free PMC article. Review.
-
Paclitaxel and docetaxel in prostate cancer.Hematol Oncol Clin North Am. 2001 Jun;15(3):525-45. doi: 10.1016/s0889-8588(05)70230-6. Hematol Oncol Clin North Am. 2001. PMID: 11525295 Review.
Cited by
-
ß3 integrin modulates transforming growth factor beta induced (TGFBI) function and paclitaxel response in ovarian cancer cells.Mol Cancer. 2012 May 28;11:36. doi: 10.1186/1476-4598-11-36. Mol Cancer. 2012. PMID: 22640878 Free PMC article.
-
Platinum-resistance in epithelial ovarian cancer: an interplay of epithelial-mesenchymal transition interlinked with reprogrammed metabolism.J Transl Med. 2022 Dec 3;20(1):556. doi: 10.1186/s12967-022-03776-y. J Transl Med. 2022. PMID: 36463238 Free PMC article.
-
Denaturation and solvent effect on the conformation and fibril formation of TGFBIp.Mol Vis. 2009 Dec 8;15:2617-26. Mol Vis. 2009. PMID: 20011632 Free PMC article.
-
In-cytoplasm mitochondrial transplantation for mesenchymal stem cells engineering and tissue regeneration.Bioeng Transl Med. 2021 Sep 28;7(1):e10250. doi: 10.1002/btm2.10250. eCollection 2022 Jan. Bioeng Transl Med. 2021. PMID: 35111950 Free PMC article.
-
Soluble TGFBI aggravates the malignancy of cholangiocarcinoma through activation of the ITGB1 dependent PPARγ signalling pathway.Cell Oncol (Dordr). 2022 Apr;45(2):275-291. doi: 10.1007/s13402-022-00668-7. Epub 2022 Mar 31. Cell Oncol (Dordr). 2022. PMID: 35357655
References
-
- Alli E., Bash-Babula J., Yang J.M., Hait W.N. Effect of stathmin on the sensitivity to antimicrotubule drugs in human breast cancer. Cancer Res. 2002;62:6864–6869. - PubMed
-
- Anand S., Penrhyn-Lowe S., Venkitaraman A.R. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3:51–62. - PubMed
-
- Baldassarre G., Belletti B., Nicoloso M.S., Schiappacassi M., Vecchione A., Spessotto P., Morrione A., Canzonieri V., Colombatti A. p27(Kip1)-stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell. 2005;7:51–63. - PubMed
-
- Barlow S.B., Gonzalez-Garay M.L., Cabral F. Paclitaxel-dependent mutants have severely reduced microtubule assembly and reduced tubulin synthesis. J. Cell Sci. 2002;115:3469–3478. - PubMed
-
- Billings P.C., Whitbeck J.C., Adams C.S., Abrams W.R., Cohen A.J., Engelsberg B.N., Howard P.S., Rosenbloom J. The transforming growth factor-beta-inducible matrix protein (beta)ig-h3 interacts with fibronectin. J. Biol. Chem. 2002;277:28003–28009. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

