Genetic therapies against HIV
- PMID: 18066041
- PMCID: PMC4539027
- DOI: 10.1038/nbt1367
Genetic therapies against HIV
Abstract
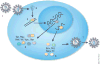
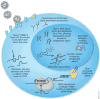
Highly active antiretroviral therapy prolongs the life of HIV-infected individuals, but it requires lifelong treatment and results in cumulative toxicities and viral-escape mutants. Gene therapy offers the promise of preventing progressive HIV infection by sustained interference with viral replication in the absence of chronic chemotherapy. Gene-targeting strategies are being developed with RNA-based agents, such as ribozymes, antisense, RNA aptamers and small interfering RNA, and protein-based agents, such as the mutant HIV Rev protein M10, fusion inhibitors and zinc-finger nucleases. Recent advances in T-cell-based strategies include gene-modified HIV-resistant T cells, lentiviral gene delivery, CD8(+) T cells, T bodies and engineered T-cell receptors. HIV-resistant hematopoietic stem cells have the potential to protect all cell types susceptible to HIV infection. The emergence of viral resistance can be addressed by therapies that use combinations of genetic agents and that inhibit both viral and host targets. Many of these strategies are being tested in ongoing and planned clinical trials.
Conflict of interest statement
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at
Figures

Similar articles
-
Novel cell and gene therapies for HIV.Cold Spring Harb Perspect Med. 2012 Oct 1;2(10):a007179. doi: 10.1101/cshperspect.a007179. Cold Spring Harb Perspect Med. 2012. PMID: 23028130 Free PMC article. Review.
-
Gene therapy for HIV infection.Expert Opin Biol Ther. 2015 Mar;15(3):319-27. doi: 10.1517/14712598.2015.967208. Epub 2014 Oct 17. Expert Opin Biol Ther. 2015. PMID: 25323559 Review.
-
RNAi as a treatment for HIV-1 infection.Biotechniques. 2006 Apr;Suppl:25-9. doi: 10.2144/000112167. Biotechniques. 2006. PMID: 16629384 Review.
-
Gene-based immunotherapy for human immunodeficiency virus infection and acquired immunodeficiency syndrome.Hum Gene Ther. 2006 Jun;17(6):577-88. doi: 10.1089/hum.2006.17.577. Hum Gene Ther. 2006. PMID: 16776567 Review.
-
Anti-HIV-1 gene expressing lentiviral vectors as an adjunctive therapy for HIV-1 infection.Curr HIV Res. 2004 Apr;2(2):185-91. doi: 10.2174/1570162043484906. Curr HIV Res. 2004. PMID: 15078182 Review.
Cited by
-
High-affinity RNA Aptamers Against the HIV-1 Protease Inhibit Both In Vitro Protease Activity and Late Events of Viral Replication.Mol Ther Nucleic Acids. 2015 Feb 17;4(2):e228. doi: 10.1038/mtna.2015.1. Mol Ther Nucleic Acids. 2015. PMID: 25689224 Free PMC article.
-
Lentiviral gene therapy against human immunodeficiency virus type 1, using a novel human TRIM21-cyclophilin A restriction factor.Hum Gene Ther. 2012 Nov;23(11):1176-85. doi: 10.1089/hum.2012.083. Epub 2012 Sep 26. Hum Gene Ther. 2012. PMID: 22909012 Free PMC article.
-
Engineering antigen-specific T cells from genetically modified human hematopoietic stem cells in immunodeficient mice.PLoS One. 2009 Dec 7;4(12):e8208. doi: 10.1371/journal.pone.0008208. PLoS One. 2009. PMID: 19997617 Free PMC article.
-
Quantitative analysis of condensation/decondensation status of pDNA in the nuclear sub-domains by QD-FRET.Nucleic Acids Res. 2011 Apr;39(7):e48. doi: 10.1093/nar/gkq1327. Epub 2011 Feb 1. Nucleic Acids Res. 2011. PMID: 21288880 Free PMC article.
-
The utility of the new generation of humanized mice to study HIV-1 infection: transmission, prevention, pathogenesis, and treatment.Retrovirology. 2011 Aug 11;8:65. doi: 10.1186/1742-4690-8-65. Retrovirology. 2011. PMID: 21835012 Free PMC article. Review.
References
-
- Sarver N, et al. Ribozymes as potential anti-HIV-1 therapeutic agents. Science. 1990;247:1222–1225. - PubMed
-
- Michienzi A, et al. RNA-mediated inhibition of HIV in a gene therapy setting. Ann NY Acad Sci. 2003;1002:63–71. - PubMed
-
- Ngok FK, et al. Clinical gene therapy research utilizing ribozymes: application to the treatment of HIV/AIDS. Methods Mol Biol. 2004;252:581–598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

