Negative feedback regulation of RIG-I-mediated antiviral signaling by interferon-induced ISG15 conjugation
- PMID: 18057259
- PMCID: PMC2224411
- DOI: 10.1128/JVI.01650-07
Negative feedback regulation of RIG-I-mediated antiviral signaling by interferon-induced ISG15 conjugation
Abstract
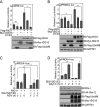
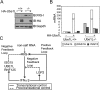
RIG-I senses intracellular virus-specific nucleic acid structures and initiates an antiviral response that induces interferon (IFN) production, which, in turn, activates the transcription of RIG-I to increase RIG-I protein levels. Upon intracellular poly(I:C) stimulation, however, the levels of RIG-I protein did not correlate with the expression patterns of RIG-I transcripts. When the ISG15 conjugation system was overexpressed, ISG15 was conjugated to RIG-I and cellular levels of the unconjugated form of RIG-I decreased. The ISGylation of RIG-I reduced levels of both basal and virus-induced IFN promoter activity. Levels of unconjugated RIG-I also decreased when 26S proteasome activity was blocked by treatment with MG132, ALLN, or Lactacystin. In the presence of MG132, ISG15 conjugation to RIG-I increased, and hence, the unconjugated form of RIG-I was reduced. In Ube1L(-/-) cells, which lack the ability to conjugate ISG15, basal levels of both RIG-I protein and transcripts were increased compared to those in wild-type cells. As a result, enhanced production of ISGs and enhanced IFN promoter activity in Ube1L(-/-) cells were observed, and the phenotype was restored to that of wild-type cells by the overexpression of Ube1L. Based on these results, we propose a novel negative feedback loop which adjusts the strength of the RIG-I-mediated antiviral response and IFN production through the regulation of RIG-I protein by IFN-induced ISG15 conjugation.
Figures




Similar articles
-
RIG-I regulates myeloid differentiation by promoting TRIM25-mediated ISGylation.Proc Natl Acad Sci U S A. 2020 Jun 23;117(25):14395-14404. doi: 10.1073/pnas.1918596117. Epub 2020 Jun 8. Proc Natl Acad Sci U S A. 2020. PMID: 32513696 Free PMC article.
-
Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways.Proc Natl Acad Sci U S A. 2005 Jul 19;102(29):10200-5. doi: 10.1073/pnas.0504754102. Epub 2005 Jul 11. Proc Natl Acad Sci U S A. 2005. PMID: 16009940 Free PMC article.
-
Involvement of UBE1L in ISG15 conjugation during retinoid-induced differentiation of acute promyelocytic leukemia.J Biol Chem. 2004 Apr 30;279(18):18178-87. doi: 10.1074/jbc.M309259200. Epub 2004 Feb 18. J Biol Chem. 2004. PMID: 14976209
-
The antiviral activities of ISG15.J Mol Biol. 2013 Dec 13;425(24):4995-5008. doi: 10.1016/j.jmb.2013.09.041. Epub 2013 Oct 3. J Mol Biol. 2013. PMID: 24095857 Free PMC article. Review.
-
Viral Evasion of RIG-I-Like Receptor-Mediated Immunity through Dysregulation of Ubiquitination and ISGylation.Viruses. 2021 Jan 26;13(2):182. doi: 10.3390/v13020182. Viruses. 2021. PMID: 33530371 Free PMC article. Review.
Cited by
-
Neuro-invasion by a 'Trojan Horse' strategy and vasculopathy during intrauterine flavivirus infection.Int J Exp Pathol. 2012 Feb;93(1):24-33. doi: 10.1111/j.1365-2613.2011.00795.x. Int J Exp Pathol. 2012. PMID: 22264283 Free PMC article.
-
Unveiling the Multifaceted Roles of ISG15: From Immunomodulation to Therapeutic Frontiers.Vaccines (Basel). 2024 Feb 1;12(2):153. doi: 10.3390/vaccines12020153. Vaccines (Basel). 2024. PMID: 38400136 Free PMC article. Review.
-
Molecular Insights into Crimean-Congo Hemorrhagic Fever Virus.Viruses. 2016 Apr 21;8(4):106. doi: 10.3390/v8040106. Viruses. 2016. PMID: 27110812 Free PMC article. Review.
-
ISG15 and immune diseases.Biochim Biophys Acta. 2010 May;1802(5):485-96. doi: 10.1016/j.bbadis.2010.02.006. Epub 2010 Feb 12. Biochim Biophys Acta. 2010. PMID: 20153823 Free PMC article. Review.
-
Viral hijacking of the host ubiquitin system to evade interferon responses.Curr Opin Microbiol. 2010 Aug;13(4):517-23. doi: 10.1016/j.mib.2010.05.012. Epub 2010 Jun 17. Curr Opin Microbiol. 2010. PMID: 20699190 Free PMC article. Review.
References
-
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4499-511. - PubMed
-
- Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124783-801. - PubMed
-
- Blomstrom, D. C., D. Fahey, R. Kutny, B. D. Korant, and E. Knight, Jr. 1986. Molecular characterization of the interferon-induced 15-kDa protein. Molecular cloning and nucleotide and amino acid sequence. J. Biol. Chem. 2618811-8816. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

