Functional analysis of the murine cytomegalovirus chemokine receptor homologue M33: ablation of constitutive signaling is associated with an attenuated phenotype in vivo
- PMID: 18057236
- PMCID: PMC2258698
- DOI: 10.1128/JVI.02550-06
Functional analysis of the murine cytomegalovirus chemokine receptor homologue M33: ablation of constitutive signaling is associated with an attenuated phenotype in vivo
Abstract
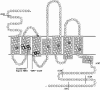
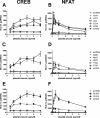
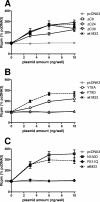
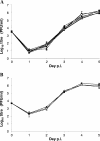
The murine cytomegalovirus (MCMV) M33 gene is conserved among all betaherpesviruses and encodes a homologue of seven-transmembrane receptors (7TMR) with the capacity for constitutive signaling. Previous studies have demonstrated that M33 is important for MCMV dissemination to or replication within the salivary glands. In this study, we probed N- and C-terminal regions of M33 as well as known 7TMR signature motifs in transmembrane (TM) II and TM III to determine the impact on cell surface expression, constitutive signaling, and in vivo phenotype. The region between amino acids R(340) and A(353) of the C terminus was found to be important for CREB- and NFAT-mediated signaling, although not essential for phosphatidylinositol turnover. Tagging or truncation of the N terminus of M33 resulted in loss of cell surface expression. Within TM II, an F79D mutation abolished constitutive signaling, demonstrating a role, as in other cellular and viral 7TMR, of TM II in receptor activation. In TM III, the arginine (but not the asparagine) residue of the NRY motif (the counterpart of the common DRY motif in cellular 7TMR) was found to be essential for constitutive signaling. Selected mutations incorporated into recombinant MCMV showed that disruption of constitutive signaling for a viral 7TMR homologue resulted in a reduced capacity to disseminate to or replicate in the salivary glands. In addition, HCMV UL33 was found to partially compensate for the lack of M33 in vivo, suggesting conserved biological roles of the UL33 gene family.
Figures
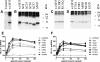
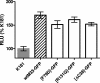
Similar articles
-
The Mouse Cytomegalovirus G Protein-Coupled Receptor Homolog, M33, Coordinates Key Features of In Vivo Infection via Distinct Components of Its Signaling Repertoire.J Virol. 2022 Feb 23;96(4):e0186721. doi: 10.1128/JVI.01867-21. Epub 2021 Dec 8. J Virol. 2022. PMID: 34878888 Free PMC article.
-
The M33 chemokine receptor homolog of murine cytomegalovirus exhibits a differential tissue-specific role during in vivo replication and latency.J Virol. 2009 Aug;83(15):7590-601. doi: 10.1128/JVI.00386-09. Epub 2009 May 13. J Virol. 2009. PMID: 19439478 Free PMC article.
-
Murine cytomegalovirus (CMV) M33 and human CMV US28 receptors exhibit similar constitutive signaling activities.J Virol. 2002 Aug;76(16):8161-8. doi: 10.1128/jvi.76.16.8161-8168.2002. J Virol. 2002. PMID: 12134021 Free PMC article.
-
Virus-encoded 7 transmembrane receptors.Prog Mol Biol Transl Sci. 2015;129:353-93. doi: 10.1016/bs.pmbts.2014.10.010. Epub 2014 Dec 24. Prog Mol Biol Transl Sci. 2015. PMID: 25595810 Review.
-
New paradigms in chemokine receptor signal transduction: Moving beyond the two-site model.Biochem Pharmacol. 2016 Aug 15;114:53-68. doi: 10.1016/j.bcp.2016.04.007. Epub 2016 Apr 19. Biochem Pharmacol. 2016. PMID: 27106080 Free PMC article. Review.
Cited by
-
Structural Diversity in Conserved Regions Like the DRY-Motif among Viral 7TM Receptors-A Consequence of Evolutionary Pressure?Adv Virol. 2012;2012:231813. doi: 10.1155/2012/231813. Epub 2012 Jul 30. Adv Virol. 2012. PMID: 22899926 Free PMC article.
-
The Mouse Cytomegalovirus G Protein-Coupled Receptor Homolog, M33, Coordinates Key Features of In Vivo Infection via Distinct Components of Its Signaling Repertoire.J Virol. 2022 Feb 23;96(4):e0186721. doi: 10.1128/JVI.01867-21. Epub 2021 Dec 8. J Virol. 2022. PMID: 34878888 Free PMC article.
-
The CMV-encoded G protein-coupled receptors M33 and US28 play pleiotropic roles in immune evasion and alter host T cell responses.Front Immunol. 2022 Dec 7;13:1047299. doi: 10.3389/fimmu.2022.1047299. eCollection 2022. Front Immunol. 2022. PMID: 36569845 Free PMC article.
-
Human Cytomegalovirus-Encoded G Protein-Coupled Receptor UL33 Facilitates Virus Dissemination via the Extracellular and Cell-to-Cell Route.Viruses. 2020 May 30;12(6):594. doi: 10.3390/v12060594. Viruses. 2020. PMID: 32486172 Free PMC article.
-
The carboxy-terminal tail of human cytomegalovirus (HCMV) US28 regulates both chemokine-independent and chemokine-dependent signaling in HCMV-infected cells.J Virol. 2009 Oct;83(19):10016-27. doi: 10.1128/JVI.00354-09. Epub 2009 Jul 15. J Virol. 2009. PMID: 19605482 Free PMC article.
References
-
- Ballesteros, J., S. Kitanovic, F. Guarnieri, P. Davies, B. J. Fromme, K. Konvicka, L. Chi, R. P. Millar, J. S. Davidson, H. Weinstein, and S. C. Sealfon. 1998. Functional microdomains in G protein-coupled receptors. The conserved arginine-cage motif in the gonadotropin-releasing hormone receptor. J. Biol. Chem. 27310445-10453. - PubMed
-
- Ballesteros, J. A., A. D. Jensen, G. Liapakis, S. G. Rasmussen, L. Shi, U. Gether, and J. A. Javitch. 2001. Activation of the beta 2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J. Biol. Chem. 27629171-29177. - PubMed
-
- Bentz, G. L., M. Jarquin-Pardo, G. Chan, M. S. Smith, C. Sinzger, and A. D. Yurochko. 2006. Human cytomegalovirus (HCMV) infection of endothelial cells promotes naive monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV. J. Virol. 8011539-11555. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

