Secreted NS1 of dengue virus attaches to the surface of cells via interactions with heparan sulfate and chondroitin sulfate E
- PMID: 18052531
- PMCID: PMC2092380
- DOI: 10.1371/journal.ppat.0030183
Secreted NS1 of dengue virus attaches to the surface of cells via interactions with heparan sulfate and chondroitin sulfate E
Abstract
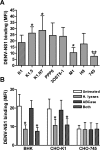
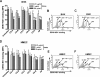
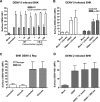
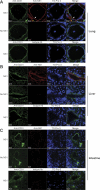
Dengue virus (DENV) nonstructural protein-1 (NS1) is a secreted glycoprotein that is absent from viral particles but accumulates in the supernatant and on the plasma membrane of cells during infection. Immune recognition of cell surface NS1 on endothelial cells has been hypothesized as a mechanism for the vascular leakage that occurs during severe DENV infection. However, it has remained unclear how NS1 becomes associated with the plasma membrane, as it contains no membrane-spanning sequence motif. Using flow cytometric and ELISA-based binding assays and mutant cell lines lacking selective glycosaminoglycans, we show that soluble NS1 binds back to the surface of uninfected cells primarily via interactions with heparan sulfate and chondroitin sulfate E. DENV NS1 binds directly to the surface of many types of epithelial and mesenchymal cells yet attaches poorly to most peripheral blood cells. Moreover, DENV NS1 preferentially binds to cultured human microvascular compared to aortic or umbilical cord vein endothelial cells. This binding specificity was confirmed in situ as DENV NS1 bound to lung and liver but not intestine or brain endothelium of mouse tissues. Differential binding of soluble NS1 by tissue endothelium and subsequent recognition by anti-NS1 antibodies could contribute to the selective vascular leakage syndrome that occurs during severe secondary DENV infection.
Conflict of interest statement
Figures

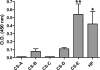


Similar articles
-
Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability.PLoS Pathog. 2016 Jul 14;12(7):e1005738. doi: 10.1371/journal.ppat.1005738. eCollection 2016 Jul. PLoS Pathog. 2016. PMID: 27416066 Free PMC article.
-
Dual targeting of dengue virus virions and NS1 protein with the heparan sulfate mimic PG545.Antiviral Res. 2019 Aug;168:121-127. doi: 10.1016/j.antiviral.2019.05.004. Epub 2019 May 11. Antiviral Res. 2019. PMID: 31085206
-
Dengue virus non-structural protein 1 activates the p38 MAPK pathway to decrease barrier integrity in primary human endothelial cells.J Gen Virol. 2020 May;101(5):484-496. doi: 10.1099/jgv.0.001401. Epub 2020 Mar 4. J Gen Virol. 2020. PMID: 32141809
-
The Good, the Bad, and the Shocking: The Multiple Roles of Dengue Virus Nonstructural Protein 1 in Protection and Pathogenesis.Annu Rev Virol. 2018 Sep 29;5(1):227-253. doi: 10.1146/annurev-virology-101416-041848. Epub 2018 Jul 25. Annu Rev Virol. 2018. PMID: 30044715 Free PMC article. Review.
-
Dengue virus non-structural protein 1: a pathogenic factor, therapeutic target, and vaccine candidate.J Biomed Sci. 2018 Jul 24;25(1):58. doi: 10.1186/s12929-018-0462-0. J Biomed Sci. 2018. PMID: 30037331 Free PMC article. Review.
Cited by
-
ApoA1 Neutralizes Proinflammatory Effects of Dengue Virus NS1 Protein and Modulates Viral Immune Evasion.J Virol. 2021 Jun 10;95(13):e0197420. doi: 10.1128/JVI.01974-20. Epub 2021 Jun 10. J Virol. 2021. PMID: 33827950 Free PMC article.
-
Roles for endothelial cells in dengue virus infection.Adv Virol. 2012;2012:840654. doi: 10.1155/2012/840654. Epub 2012 Aug 16. Adv Virol. 2012. PMID: 22952474 Free PMC article.
-
The Complexity of a Dengue Vaccine: A Review of the Human Antibody Response.PLoS Negl Trop Dis. 2015 Jun 11;9(6):e0003749. doi: 10.1371/journal.pntd.0003749. eCollection 2015. PLoS Negl Trop Dis. 2015. PMID: 26065421 Free PMC article. Review.
-
Complement and its role in protection and pathogenesis of flavivirus infections.Vaccine. 2008 Dec 30;26 Suppl 8:I100-7. doi: 10.1016/j.vaccine.2008.11.061. Vaccine. 2008. PMID: 19388173 Free PMC article. Review.
-
Cardiovascular manifestations of the emerging dengue pandemic.Nat Rev Cardiol. 2014 Jun;11(6):335-45. doi: 10.1038/nrcardio.2014.40. Epub 2014 Apr 8. Nat Rev Cardiol. 2014. PMID: 24710495 Review.
References
-
- Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social, and economic problem in the 21st century. Trends Microbiol. 2002;10:100–103. - PubMed
-
- Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, et al. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol. 1984;120:653–669. - PubMed
-
- Guzman MG, Kouri G, Valdes L, Bravo J, Alvarez M, et al. Epidemiologic studies on dengue in Santiago de Cuba, 1997. Am J Epidemiol. 2000;152:793–799. 804. discussion. - PubMed
-
- Nimmannitya S. Clinical spectrum and management of dengue haemorrhagic fever. Southeast Asian J Trop Med Public Health. 1987;18:392–397. - PubMed
-
- Rothman AL, Ennis FA. Immunopathogenesis of dengue hemorrhagic fever. Virology. 1999;257:1–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

