Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues
- PMID: 18045540
- PMCID: PMC2330270
- DOI: 10.1016/j.cell.2007.09.047
Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues
Abstract
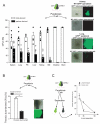
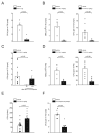
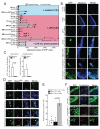
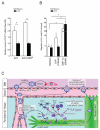
Constitutive egress of bone marrow (BM)-resident hematopoietic stem and progenitor cells (HSPCs) into the blood is a well-established phenomenon, but the ultimate fate and functional relevance of circulating HSPCs is largely unknown. We show that mouse thoracic duct (TD) lymph contains HSPCs that possess short- and long-term multilineage reconstitution capacity. TD-derived HSPCs originate in the BM, enter the blood, and traffic to multiple peripheral organs, where they reside for at least 36 hr before entering draining lymphatics to return to the blood and, eventually, the BM. HSPC egress from extramedullary tissues into lymph depends on sphingosine-1-phosphate receptors. Migratory HSPCs proliferate within extramedullary tissues and give rise to tissue-resident myeloid cells, preferentially dendritic cells. HSPC differentiation is amplified upon exposure to Toll-like receptor agonists. Thus, HSPCs can survey peripheral organs and can foster the local production of tissue-resident innate immune cells under both steady-state conditions and in response to inflammatory signals.
Figures



Comment in
-
Stem cells on patrol.Cell. 2007 Nov 30;131(5):842-4. doi: 10.1016/j.cell.2007.11.010. Cell. 2007. PMID: 18045530 Free PMC article.
Similar articles
-
Novel trafficking routes for hematopoietic stem and progenitor cells.Ann N Y Acad Sci. 2009 Sep;1176:87-93. doi: 10.1111/j.1749-6632.2009.04609.x. Ann N Y Acad Sci. 2009. PMID: 19796236 Free PMC article.
-
Different Human Immune Lineage Compositions Are Generated in Non-Conditioned NBSGW Mice Depending on HSPC Source.Front Immunol. 2020 Oct 19;11:573406. doi: 10.3389/fimmu.2020.573406. eCollection 2020. Front Immunol. 2020. PMID: 33193358 Free PMC article.
-
Sphingosine-1-Phosphate Receptor-3 Supports Hematopoietic Stem and Progenitor Cell Residence Within the Bone Marrow Niche.Stem Cells. 2017 Apr;35(4):1040-1052. doi: 10.1002/stem.2556. Epub 2017 Jan 19. Stem Cells. 2017. PMID: 28026131 Free PMC article.
-
Hematopoietic stem and progenitor cells: their mobilization and homing to bone marrow and peripheral tissue.Immunol Res. 2009;44(1-3):160-8. doi: 10.1007/s12026-009-8109-6. Immunol Res. 2009. PMID: 19340403 Review.
-
Trafficking of murine hematopoietic stem and progenitor cells in health and vascular disease.Microcirculation. 2009 Aug;16(6):497-507. doi: 10.1080/10739680902948351. Epub 2009 May 26. Microcirculation. 2009. PMID: 19479622 Review.
Cited by
-
Linking stem cells to chromosomal instability.Oncoimmunology. 2012 Mar 1;1(2):195-200. doi: 10.4161/onci.1.2.18613. Oncoimmunology. 2012. PMID: 22720241 Free PMC article.
-
Shaping the landscape: metabolic regulation of S1P gradients.Biochim Biophys Acta. 2013 Jan;1831(1):193-202. doi: 10.1016/j.bbalip.2012.06.007. Epub 2012 Jun 23. Biochim Biophys Acta. 2013. PMID: 22735358 Free PMC article. Review.
-
CD34+DNAM-1brightCXCR4+ haemopoietic precursors circulate after chemotherapy, seed lung tissue and generate functional innate-like T cells and NK cells.Front Immunol. 2024 Feb 8;15:1332781. doi: 10.3389/fimmu.2024.1332781. eCollection 2024. Front Immunol. 2024. PMID: 38390333 Free PMC article.
-
Extramedullary myelopoiesis in malaria depends on mobilization of myeloid-restricted progenitors by IFN-γ induced chemokines.PLoS Pathog. 2013;9(6):e1003406. doi: 10.1371/journal.ppat.1003406. Epub 2013 Jun 6. PLoS Pathog. 2013. PMID: 23762028 Free PMC article.
-
Mobilization of hematopoietic stem/progenitor cells: general principles and molecular mechanisms.Methods Mol Biol. 2012;904:1-14. doi: 10.1007/978-1-61779-943-3_1. Methods Mol Biol. 2012. PMID: 22890918 Free PMC article. Review.
References
-
- Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. - PubMed
-
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. - PubMed
-
- Cardier JE, Barbera-Guillem E. Extramedullary hematopoiesis in the adult mouse liver is associated with specific hepatic sinusoidal endothelial cells. Hepatology. 1997;26:165–175. - PubMed
-
- Chiba K, Yanagawa Y, Masubuchi Y, Kataoka H, Kawaguchi T, Ohtsuki M, Hoshino Y. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol. 1998;160:5037–5044. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR42689/AR/NIAMS NIH HHS/United States
- R01 AI061663-05/AI/NIAID NIH HHS/United States
- HL56949/HL/NHLBI NIH HHS/United States
- S10 RR021190/RR/NCRR NIH HHS/United States
- P01 HL056949-10/HL/NHLBI NIH HHS/United States
- P01 HL056949-08/HL/NHLBI NIH HHS/United States
- P30 AR042689/AR/NIAMS NIH HHS/United States
- P01 HL056949-119001/HL/NHLBI NIH HHS/United States
- P01 HL056949-089001/HL/NHLBI NIH HHS/United States
- R01 AI072252-01/AI/NIAID NIH HHS/United States
- P01 HL056949/HL/NHLBI NIH HHS/United States
- R01 AI069259-03/AI/NIAID NIH HHS/United States
- P01 HL056949-09/HL/NHLBI NIH HHS/United States
- P01 HL056949-129001/HL/NHLBI NIH HHS/United States
- R01 AI061663/AI/NIAID NIH HHS/United States
- AI061663/AI/NIAID NIH HHS/United States
- P01 HL056949-11/HL/NHLBI NIH HHS/United States
- S10 RR021190-010001/RR/NCRR NIH HHS/United States
- P01 HL056949-109001/HL/NHLBI NIH HHS/United States
- R01 AI072252/AI/NIAID NIH HHS/United States
- R01 AI069259/AI/NIAID NIH HHS/United States
- P01 HL056949-099001/HL/NHLBI NIH HHS/United States
- P01 HL056949-12/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

