Localization of HIV-1 Vpr to the nuclear envelope: impact on Vpr functions and virus replication in macrophages
- PMID: 18039376
- PMCID: PMC2211753
- DOI: 10.1186/1742-4690-4-84
Localization of HIV-1 Vpr to the nuclear envelope: impact on Vpr functions and virus replication in macrophages
Abstract
Background: HIV-1 Vpr is a dynamic protein that primarily localizes in the nucleus, but a significant fraction is concentrated at the nuclear envelope (NE), supporting an interaction between Vpr and components of the nuclear pore complex, including the nucleoporin hCG1. In the present study, we have explored the contribution of Vpr accumulation at the NE to the Vpr functions, including G2-arrest and pro-apoptotic activities, and virus replication in primary macrophages.
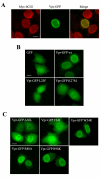
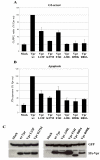
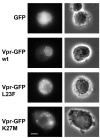
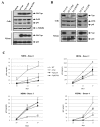
Results: In order to define the functional role of Vpr localization at the NE, we have characterized a set of single-point Vpr mutants, and selected two new mutants with substitutions within the first alpha-helix of the protein, Vpr-L23F and Vpr-K27M, that failed to associate with hCG1, but were still able to interact with other known relevant host partners of Vpr. In mammalian cells, these mutants failed to localize at the NE resulting in a diffuse nucleocytoplasmic distribution both in HeLa cells and in primary human monocyte-derived macrophages. Other mutants with substitutions in the first alpha-helix (Vpr-A30L and Vpr-F34I) were similarly distributed between the nucleus and cytoplasm, demonstrating that this helix contains the determinants required for localization of Vpr at the NE. All these mutations also impaired the Vpr-mediated G2-arrest of the cell cycle and the subsequent cell death induction, indicating a functional link between these activities and the Vpr accumulation at the NE. However, this localization is not sufficient, since mutations within the C-terminal basic region of Vpr (Vpr-R80A and Vpr-R90K), disrupted the G2-arrest and apoptotic activities without altering NE localization. Finally, the replication of the Vpr-L23F and Vpr-K27M hCG1-binding deficient mutant viruses was also affected in primary macrophages from some but not all donors.
Conclusion: These results indicate that the targeting of Vpr to the nuclear pore complex may constitute an early step toward Vpr-induced G2-arrest and subsequent apoptosis; they also suggest that Vpr targeting to the nuclear pore complex is not absolutely required, but can improve HIV-1 replication in macrophages.
Figures


Similar articles
-
Docking of HIV-1 Vpr to the nuclear envelope is mediated by the interaction with the nucleoporin hCG1.J Biol Chem. 2002 Nov 22;277(47):45091-8. doi: 10.1074/jbc.M207439200. Epub 2002 Sep 12. J Biol Chem. 2002. PMID: 12228227
-
Nuclear import, virion incorporation, and cell cycle arrest/differentiation are mediated by distinct functional domains of human immunodeficiency virus type 1 Vpr.J Virol. 1997 Sep;71(9):6339-47. doi: 10.1128/JVI.71.9.6339-6347.1997. J Virol. 1997. PMID: 9261351 Free PMC article.
-
Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr.Science. 2001 Nov 2;294(5544):1105-8. doi: 10.1126/science.1063957. Science. 2001. PMID: 11691994
-
Role of Vpr in HIV-1 nuclear import: therapeutic implications.Curr HIV Res. 2009 Mar;7(2):136-43. doi: 10.2174/157016209787581418. Curr HIV Res. 2009. PMID: 19275582 Review.
-
HIV-1 Vpr: G2 cell cycle arrest, macrophages and nuclear transport.Prog Cell Cycle Res. 1997;3:21-7. doi: 10.1007/978-1-4615-5371-7_2. Prog Cell Cycle Res. 1997. PMID: 9552403 Review.
Cited by
-
Roles of Vpr and Vpx in modulating the virus-host cell relationship.Mol Aspects Med. 2010 Oct;31(5):398-406. doi: 10.1016/j.mam.2010.05.002. Epub 2010 Jun 15. Mol Aspects Med. 2010. PMID: 20558198 Free PMC article. Review.
-
Analysis of the viral elements required in the nuclear import of HIV-1 DNA.J Virol. 2010 Jan;84(2):729-39. doi: 10.1128/JVI.01952-09. Epub 2009 Nov 4. J Virol. 2010. PMID: 19889772 Free PMC article.
-
Macrophages: Key Cellular Players in HIV Infection and Pathogenesis.Viruses. 2024 Feb 13;16(2):288. doi: 10.3390/v16020288. Viruses. 2024. PMID: 38400063 Free PMC article. Review.
-
Limelight on two HIV/SIV accessory proteins in macrophage infection: is Vpx overshadowing Vpr?Retrovirology. 2010 Apr 9;7:35. doi: 10.1186/1742-4690-7-35. Retrovirology. 2010. PMID: 20380700 Free PMC article. Review.
-
Molecular and functional basis for the scaffolding role of the p50/dynamitin subunit of the microtubule-associated dynactin complex.J Biol Chem. 2010 Jul 23;285(30):23019-31. doi: 10.1074/jbc.M110.100602. Epub 2010 May 12. J Biol Chem. 2010. PMID: 20463029 Free PMC article.
References
-
- Balliet JW, Kolson DL, Eiger G, Kim FM, McGann KA, Srinivasan A, Collman R. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology. 1994;200:623–31. doi: 10.1006/viro.1994.1225. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

