HSV-mediated transfer of interleukin-10 reduces inflammatory pain through modulation of membrane tumor necrosis factor alpha in spinal cord microglia
- PMID: 18033311
- PMCID: PMC2572752
- DOI: 10.1038/sj.gt.3303054
HSV-mediated transfer of interleukin-10 reduces inflammatory pain through modulation of membrane tumor necrosis factor alpha in spinal cord microglia
Abstract
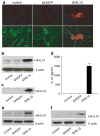
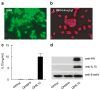
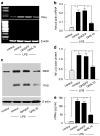
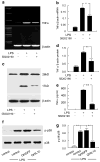
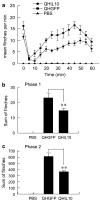
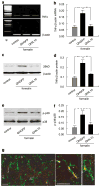
To dissect the molecular basis of the neuroimmune response associated with the genesis of inflammatory (nociceptive) pain, we constructed a herpes simplex virus-based gene transfer vector to express the antiinflammatory cytokine interleukin-10 (IL-10), and used it to examine the effect of IL-10 expression in activated microglial cells in vitro, and in inflammatory pain in vivo. IL-10 reduced the phosphorylation of p38 mitogen-activated protein kinase (MAPK) and decreased the expression of full-length membrane spanning tumor necrosis factor-alpha (mTNFalpha) following lipopolysaccharide stimulation of microglia in vitro. IL-10 also reduced intracellular cleavage of mTNFalpha and release of the soluble cleavage product sTNFalpha. Similar effects on TNFalpha expression were observed when the cells were pretreated with a p38 MAPK inhibitor. In animals, injection of a dilute solution of formalin in the skin resulted in an increase in mTNFalpha in spinal dorsal horn, without detectable sTNFalpha. Local release of IL-10 achieved by gene transfer reduced the number of spontaneous flinches in the early and delayed phases of the formalin test of inflammatory pain. The effect of IL-10 on nocisponsive behavior correlated with a block in phosphorylation of p38 and reduced expression of 26 kDa mTNFalpha in spinal microglia. The results emphasize the key role played by membrane TNFalpha in the spinal neuroimmune response in pain caused by peripheral inflammation.
Conflict of interest statement
Disclosure/conflict of interest
The authors declare no conflicts of interest.
Figures
Similar articles
-
Interleukin 10 mediated by herpes simplex virus vectors suppresses neuropathic pain induced by human immunodeficiency virus gp120 in rats.Anesth Analg. 2014 Sep;119(3):693-701. doi: 10.1213/ANE.0000000000000311. Anesth Analg. 2014. PMID: 25137003 Free PMC article.
-
Gene transfer to interfere with TNFalpha signaling in neuropathic pain.Gene Ther. 2007 Jul;14(13):1010-6. doi: 10.1038/sj.gt.3302950. Epub 2007 Apr 19. Gene Ther. 2007. PMID: 17443214
-
Herpes simplex virus vector-mediated expression of interleukin-10 reduces below-level central neuropathic pain after spinal cord injury.Neurorehabil Neural Repair. 2012 Sep;26(7):889-97. doi: 10.1177/1545968312445637. Epub 2012 May 15. Neurorehabil Neural Repair. 2012. PMID: 22593113 Free PMC article.
-
A human trial of HSV-mediated gene transfer for the treatment of chronic pain.Gene Ther. 2009 Apr;16(4):455-60. doi: 10.1038/gt.2009.17. Epub 2009 Feb 26. Gene Ther. 2009. PMID: 19242524 Free PMC article. Review.
-
Gene therapy directed at the neuroimmune component of chronic pain with particular attention to the role of TNF alpha.Neurosci Lett. 2008 Jun 6;437(3):209-13. doi: 10.1016/j.neulet.2008.03.049. Epub 2008 Mar 22. Neurosci Lett. 2008. PMID: 18403116 Free PMC article. Review.
Cited by
-
Regular physical activity prevents chronic pain by altering resident muscle macrophage phenotype and increasing interleukin-10 in mice.Pain. 2016 Jan;157(1):70-79. doi: 10.1097/j.pain.0000000000000312. Pain. 2016. PMID: 26230740 Free PMC article.
-
Interleukin 10 mediated by herpes simplex virus vectors suppresses neuropathic pain induced by human immunodeficiency virus gp120 in rats.Anesth Analg. 2014 Sep;119(3):693-701. doi: 10.1213/ANE.0000000000000311. Anesth Analg. 2014. PMID: 25137003 Free PMC article.
-
Spinal interleukin-10 therapy to treat peripheral neuropathic pain.Neuromodulation. 2012 Nov-Dec;15(6):520-6; discussion 526. doi: 10.1111/j.1525-1403.2012.00462.x. Epub 2012 Jun 1. Neuromodulation. 2012. PMID: 22672183 Free PMC article. Review.
-
The Role of Interleukin-10 in the Pathogenesis and Treatment of a Spinal Cord Injury.Diagnostics (Basel). 2024 Jan 9;14(2):151. doi: 10.3390/diagnostics14020151. Diagnostics (Basel). 2024. PMID: 38248028 Free PMC article. Review.
-
Cytochrome P450 26A1 Contributes to the Maintenance of Neuropathic Pain.Neurosci Bull. 2024 Mar;40(3):293-309. doi: 10.1007/s12264-023-01101-1. Epub 2023 Aug 28. Neurosci Bull. 2024. PMID: 37639183 Free PMC article.
References
-
- Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience. 1997;81:255–262. - PubMed
-
- Anzai H, Hamba M, Onda A, Konno S, Kikuchi S. Epidural application of nucleus pulposus enhances nociresponses of rat dorsal horn neurons. Spine. 2002;27:E50–E55. - PubMed
-
- Onda A, Hamba M, Yabuki S, Kikuchi S. Exogenous tumor necrosis factor-alpha induces abnormal discharges in rat dorsal horn neurons. Spine. 2002;27:1618–1624. discussion 1624. - PubMed
-
- Sorkin LS, Doom CM. Epineurial application of TNF elicits an acute mechanical hyperalgesia in the awake rat. J Peripher Nerv Syst. 2000;5:96–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

