Arf6 and microtubules in adhesion-dependent trafficking of lipid rafts
- PMID: 18026091
- PMCID: PMC2715295
- DOI: 10.1038/ncb1657
Arf6 and microtubules in adhesion-dependent trafficking of lipid rafts
Abstract
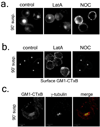
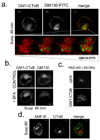
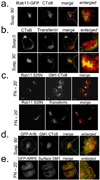
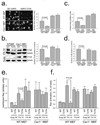
Integrin-mediated adhesion regulates membrane binding sites for Rac1 within lipid rafts. Detachment of cells from the substratum triggers the clearance of rafts from the plasma membrane through caveolin-dependent internalization. The small GTPase Arf6 and microtubules also regulate Rac-dependent cell spreading and migration, but the mechanisms are poorly understood. Here we show that endocytosis of rafts after detachment requires F-actin, followed by microtubule-dependent trafficking to recycling endosomes. When cells are replated on fibronectin, rafts exit from recycling endosomes in an Arf6-dependent manner and return to the plasma membrane along microtubules. Both of these steps are required for the plasma membrane targeting of Rac1 and for its activation. These data therefore define a new membrane raft trafficking pathway that is crucial for anchorage-dependent signalling.
Figures

Similar articles
-
RalA-exocyst complex regulates integrin-dependent membrane raft exocytosis and growth signaling.Curr Biol. 2010 Jan 12;20(1):75-9. doi: 10.1016/j.cub.2009.11.016. Epub 2009 Dec 10. Curr Biol. 2010. PMID: 20005108 Free PMC article.
-
Recycling of Raft-associated prohormone sorting receptor carboxypeptidase E requires interaction with ARF6.Mol Biol Cell. 2003 Nov;14(11):4448-57. doi: 10.1091/mbc.e02-11-0758. Epub 2003 Sep 5. Mol Biol Cell. 2003. PMID: 12960436 Free PMC article.
-
Ral-Arf6 crosstalk regulates Ral dependent exocyst trafficking and anchorage independent growth signalling.Cell Signal. 2016 Sep;28(9):1225-1236. doi: 10.1016/j.cellsig.2016.05.023. Epub 2016 Jun 4. Cell Signal. 2016. PMID: 27269287 Free PMC article.
-
Roles of lipid rafts in membrane transport.Curr Opin Cell Biol. 2001 Aug;13(4):470-7. doi: 10.1016/s0955-0674(00)00238-6. Curr Opin Cell Biol. 2001. PMID: 11454454 Review.
-
Integrin signaling and lipid rafts.Cell Cycle. 2004 Jun;3(6):725-8. Epub 2004 Jun 28. Cell Cycle. 2004. PMID: 15197344 Review.
Cited by
-
Exo70 subunit of the exocyst complex is involved in adhesion-dependent trafficking of caveolin-1.PLoS One. 2012;7(12):e52627. doi: 10.1371/journal.pone.0052627. Epub 2012 Dec 27. PLoS One. 2012. PMID: 23300727 Free PMC article.
-
Caveolae as plasma membrane sensors, protectors and organizers.Nat Rev Mol Cell Biol. 2013 Feb;14(2):98-112. doi: 10.1038/nrm3512. Nat Rev Mol Cell Biol. 2013. PMID: 23340574 Review.
-
Endocytosis and signaling: cell logistics shape the eukaryotic cell plan.Physiol Rev. 2012 Jan;92(1):273-366. doi: 10.1152/physrev.00005.2011. Physiol Rev. 2012. PMID: 22298658 Free PMC article. Review.
-
RalA-exocyst complex regulates integrin-dependent membrane raft exocytosis and growth signaling.Curr Biol. 2010 Jan 12;20(1):75-9. doi: 10.1016/j.cub.2009.11.016. Epub 2009 Dec 10. Curr Biol. 2010. PMID: 20005108 Free PMC article.
-
The endocytic matrix.Nature. 2010 Jan 28;463(7280):464-73. doi: 10.1038/nature08910. Nature. 2010. PMID: 20110990 Review.
References
-
- del Pozo MA, Alderson NB, Kiosses WB, Chiang Hui-Hsien, Anderson RGW, Schwartz MA. Integrins Regulate Rac Targeting by Internalization of Membrane Domains. Science. 2004;303:839–842. - PubMed
-
- Palazzo AF, Eng CH, Schlaepfer DD, Marcantonio EE, Gundersen GG. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science. 2004;303:836–839. - PubMed
-
- Vasanji A, Ghosh PK, Graham LM, Eppell SJ, Fox PL. Polarization of plasma membrane microviscosity during endothelial cell migration. Dev Cell. 2004;6:29–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

