Cysteinyl leukotrienes are autocrine and paracrine regulators of fibrocyte function
- PMID: 18025235
- PMCID: PMC3646370
- DOI: 10.4049/jimmunol.179.11.7883
Cysteinyl leukotrienes are autocrine and paracrine regulators of fibrocyte function
Abstract
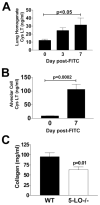
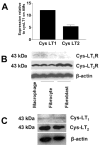
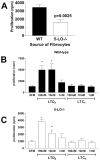
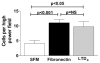
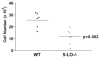
Pulmonary fibrosis is characterized by the accumulation of fibroblasts and myofibroblasts. These cells may accumulate from three potential sources: the expansion of resident lung fibroblasts, the process of epithelial-mesenchymal transition, or the recruitment and differentiation of circulating mesenchymal precursors known as fibrocytes. We have previously demonstrated that fibrocytes participate in lung fibrogenesis following administration of FITC to mice. We now demonstrate that leukotriene-deficient 5-LO(-/-) mice are protected from FITC-induced fibrosis. Both murine and human fibrocytes express both cysteinyl leukotriene receptor (CysLT) 1 and CysLT2. In addition, fibrocytes are capable of producing CysLTs and can be regulated via the autocrine or paracrine secretion of these lipid mediators. Exogenous administration of leukotriene (LT) D(4), but not LTC(4) induces proliferation of both murine and human fibrocytes in a dose-dependent manner. Consistent with this result, CysLT1 receptor antagonists are able to block the mitogenic effects of exogenous LTD(4) on fibrocytes. Endogenous production of CysLTs contributes to basal fibrocyte proliferation, but does not alter fibrocyte responses to basic fibroblast growth factor. Although CysLTs can induce the migration of fibrocytes in vitro, they do not appear to be essential for fibrocyte recruitment to the lung in vivo, possibly due to compensatory chemokine-mediated recruitment signals. However, CysLTs do appear to regulate the proliferation of fibrocytes once they are recruited to the lung. These data provide mechanistic insight into the therapeutic benefit of leukotriene synthesis inhibitors and CysLT1 receptor antagonists in animal models of fibrosis.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures




Similar articles
-
Human T(H)2 cells respond to cysteinyl leukotrienes through selective expression of cysteinyl leukotriene receptor 1.J Allergy Clin Immunol. 2012 Apr;129(4):1136-42. doi: 10.1016/j.jaci.2012.01.057. Epub 2012 Mar 3. J Allergy Clin Immunol. 2012. PMID: 22391114
-
CysLT2 receptors interact with CysLT1 receptors and down-modulate cysteinyl leukotriene dependent mitogenic responses of mast cells.Blood. 2007 Nov 1;110(9):3263-70. doi: 10.1182/blood-2007-07-100453. Epub 2007 Aug 10. Blood. 2007. PMID: 17693579 Free PMC article.
-
Eosinophils and cysteinyl leukotrienes.Prostaglandins Leukot Essent Fatty Acids. 2003 Aug-Sep;69(2-3):135-43. doi: 10.1016/s0952-3278(03)00074-7. Prostaglandins Leukot Essent Fatty Acids. 2003. PMID: 12895596 Review.
-
Functional characteristics of cysteinyl-leukotriene receptor subtypes.Life Sci. 2002 Jun 28;71(6):611-22. doi: 10.1016/s0024-3205(02)01733-2. Life Sci. 2002. PMID: 12072150 Review.
-
Functional characterisation of receptors for cysteinyl leukotrienes in smooth muscle.Acta Physiol Scand Suppl. 1998 Mar;641:1-55. Acta Physiol Scand Suppl. 1998. PMID: 9597121 Review.
Cited by
-
Prostaglandin E2 and the pathogenesis of pulmonary fibrosis.Am J Respir Cell Mol Biol. 2011 Sep;45(3):445-52. doi: 10.1165/rcmb.2011-0025RT. Epub 2011 Mar 18. Am J Respir Cell Mol Biol. 2011. PMID: 21421906 Free PMC article. Review.
-
Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis.Int J Biochem Cell Biol. 2009 Aug-Sep;41(8-9):1708-18. doi: 10.1016/j.biocel.2009.02.020. Epub 2009 Mar 6. Int J Biochem Cell Biol. 2009. PMID: 19433312 Free PMC article.
-
Fibrosis--a lethal component of systemic sclerosis.Nat Rev Rheumatol. 2014 Jul;10(7):390-402. doi: 10.1038/nrrheum.2014.53. Epub 2014 Apr 22. Nat Rev Rheumatol. 2014. PMID: 24752182 Review.
-
γ-Herpes virus-68, but not Pseudomonas aeruginosa or influenza A (H1N1), exacerbates established murine lung fibrosis.Am J Physiol Lung Cell Mol Physiol. 2014 Aug 1;307(3):L219-30. doi: 10.1152/ajplung.00300.2013. Epub 2014 May 30. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24879051 Free PMC article.
-
IL-33 enhanced the proliferation and constitutive production of IL-13 and IL-5 by fibrocytes.Biomed Res Int. 2014;2014:738625. doi: 10.1155/2014/738625. Epub 2014 Apr 13. Biomed Res Int. 2014. PMID: 24822215 Free PMC article.
References
-
- Kuhn C. Pathology. In: Phan S, Thrall R, editors. Pulmonary Fibrosis. Dekker; New York: 1995. pp. 59–83.
-
- Gottleib DJ, Snider GL. Lung function in pulmonary fibrosis. In: Phan S, Thrall R, editors. Pulmonary Fibrosis. Vol. 85 Dekker; New York: 1995.
-
- Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. - PubMed
-
- Chesney J, Bucala R. Peripheral blood fibrocytes: mesenchymal precursor cells and the pathogenesis of fibrosis. Curr Rheumatol Rep. 2000;2:501–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

