SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells
- PMID: 18022664
- PMCID: PMC2312501
- DOI: 10.1016/j.virol.2007.09.045
SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells
Abstract
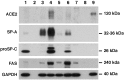
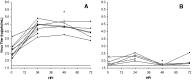
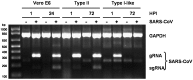
Severe acute respiratory syndrome (SARS) is a disease characterized by diffuse alveolar damage. We isolated human alveolar type II cells and maintained them in a highly differentiated state. Type II cell cultures supported SARS-CoV replication as evidenced by RT-PCR detection of viral subgenomic RNA and an increase in virus titer. Virus titers were maximal by 24 h and peaked at approximately 10(5) pfu/mL. Two cell types within the cultures were infected. One cell type was type II cells, which were positive for SP-A, SP-C, cytokeratin, a type II cell-specific monoclonal antibody, and Ep-CAM. The other cell type was composed of spindle-shaped cells that were positive for vimentin and collagen III and likely fibroblasts. Viral replication was not detected in type I-like cells or macrophages. Hence, differentiated adult human alveolar type II cells were infectible but alveolar type I-like cells and alveolar macrophages did not support productive infection.
Figures

Similar articles
-
Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus.Am J Respir Cell Mol Biol. 2013 Jun;48(6):742-8. doi: 10.1165/rcmb.2012-0339OC. Am J Respir Cell Mol Biol. 2013. PMID: 23418343 Free PMC article.
-
Viral replication and innate host responses in primary human alveolar epithelial cells and alveolar macrophages infected with influenza H5N1 and H1N1 viruses.J Virol. 2011 Jul;85(14):6844-55. doi: 10.1128/JVI.02200-10. Epub 2011 May 4. J Virol. 2011. PMID: 21543489 Free PMC article.
-
Time course and cellular localization of SARS-CoV nucleoprotein and RNA in lungs from fatal cases of SARS.PLoS Med. 2006 Feb;3(2):e27. doi: 10.1371/journal.pmed.0030027. Epub 2006 Jan 3. PLoS Med. 2006. PMID: 16379499 Free PMC article.
-
SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium.Virus Res. 2008 Apr;133(1):33-44. doi: 10.1016/j.virusres.2007.03.013. Epub 2007 Apr 23. Virus Res. 2008. PMID: 17451829 Free PMC article. Review.
-
Host-pathogen interactions during coronavirus infection of primary alveolar epithelial cells.J Leukoc Biol. 2009 Nov;86(5):1145-51. doi: 10.1189/jlb.0209078. Epub 2009 Jul 28. J Leukoc Biol. 2009. PMID: 19638499 Free PMC article. Review.
Cited by
-
The pathophysiology of SARS-CoV-2: A suggested model and therapeutic approach.Life Sci. 2020 Oct 1;258:118166. doi: 10.1016/j.lfs.2020.118166. Epub 2020 Jul 31. Life Sci. 2020. PMID: 32739471 Free PMC article. Review.
-
SARS-CoV-2 Infection and Lung Regeneration.Clin Microbiol Rev. 2022 Apr 20;35(2):e0018821. doi: 10.1128/cmr.00188-21. Epub 2022 Feb 2. Clin Microbiol Rev. 2022. PMID: 35107300 Free PMC article. Review.
-
SARS-CoV-2 (COVID-19) Adhesion Site Protein Upregulation in Small Airways, Type 2 Pneumocytes, and Alveolar Macrophages of Smokers and COPD - Possible Implications for Interstitial Fibrosis.Int J Chron Obstruct Pulmon Dis. 2022 Jan 11;17:101-115. doi: 10.2147/COPD.S329783. eCollection 2022. Int J Chron Obstruct Pulmon Dis. 2022. PMID: 35046647 Free PMC article.
-
Brief Report: Rapid Clinical Recovery From Critical Coronavirus Disease 2019 With Respiratory Failure in a Pregnant Patient Treated With IV Vasoactive Intestinal Peptide.Crit Care Explor. 2022 Jan 5;4(1):e0607. doi: 10.1097/CCE.0000000000000607. eCollection 2022 Jan. Crit Care Explor. 2022. PMID: 35018346 Free PMC article.
-
Identification of Images of COVID-19 from Chest X-rays Using Deep Learning: Comparing COGNEX VisionPro Deep Learning 1.0™ Software with Open Source Convolutional Neural Networks.SN Comput Sci. 2021;2(3):130. doi: 10.1007/s42979-021-00496-w. Epub 2021 Mar 10. SN Comput Sci. 2021. PMID: 33718884 Free PMC article.
References
-
- Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., Wang H., Shen H., Qiu L., Li Z., Geng J., Cai J., Han H., Li X., Kang W., Weng D., Liang P., Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 2004;203(2):622–630. - PMC - PubMed
-
- Fang X., Song Y., Hirsch J., Galietta L.J., Pedemonte N., Zemans R.L., Dolganov G., Verkman A.S., Matthay M.A. Contribution of CFTR to apical–basolateral fluid transport in cultured human alveolar epithelial type II cells. Am. J. Physiol., Lung Cell. Mol. Physiol. 2006;290(2):L242–L249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

