Inhibition of AMP-activated protein kinase protects pancreatic beta-cells from cytokine-mediated apoptosis and CD8+ T-cell-induced cytotoxicity
- PMID: 18003756
- PMCID: PMC6101197
- DOI: 10.2337/db07-0993
Inhibition of AMP-activated protein kinase protects pancreatic beta-cells from cytokine-mediated apoptosis and CD8+ T-cell-induced cytotoxicity
Abstract
Objective: Apoptotic destruction of insulin-producing pancreatic beta-cells is involved in the etiology of both type 1 and type 2 diabetes. AMP-activated protein kinase (AMPK) is a sensor of cellular energy charge whose sustained activation has recently been implicated in pancreatic beta-cell apoptosis and in islet cell death posttransplantation. Here, we examine the importance of beta-cell AMPK in cytokine-induced apoptosis and in the cytotoxic action of CD8(+) T-cells.
Research design and methods: Clonal MIN6 beta-cells or CD1 mouse pancreatic islets were infected with recombinant adenoviruses encoding enhanced green fluorescent protein (eGFP/null), constitutively active AMPK (AMPK-CA), or dominant-negative AMPK (AMPK-DN) and exposed or not to tumor necrosis factor-alpha, interleukin-1beta, and interferon-gamma. Apoptosis was detected by monitoring the cleavage of caspase-3 and DNA fragmentation. The cytotoxic effect of CD8(+) purified T-cells was examined against pancreatic islets from NOD mice infected with either null or the AMPK-DN-expressing adenoviruses.
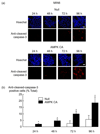
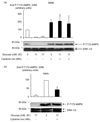
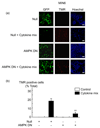
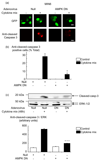
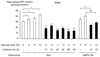
Results: Exposure to cytokines, or expression of AMPK-CA, induced apoptosis in clonal MIN6 beta-cells and CD1 mouse pancreatic islets. By contrast, overexpression of AMPK-DN protected against the proapoptotic effect of these agents, in part by preventing decreases in cellular ATP, and lowered the cytotoxic effect of CD8(+) T-cells toward NOD mouse islets.
Conclusions: Inhibition of AMPK activity enhances islet survival in the face of assault by either cytokines or T-cells. AMPK may therefore represent an interesting therapeutic target to suppress immune-mediated beta-cell destruction and may increase the efficacy of islet allografts in type 1 diabetes.
Figures
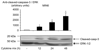


Similar articles
-
Over-expression of AMP-activated protein kinase impairs pancreatic {beta}-cell function in vivo.J Endocrinol. 2005 Nov;187(2):225-35. doi: 10.1677/joe.1.06413. J Endocrinol. 2005. PMID: 16293770
-
Suppressor of cytokine signaling-1 overexpression protects pancreatic beta cells from CD8+ T cell-mediated autoimmune destruction.J Immunol. 2004 May 1;172(9):5714-21. doi: 10.4049/jimmunol.172.9.5714. J Immunol. 2004. PMID: 15100317
-
Compound K protects pancreatic islet cells against apoptosis through inhibition of the AMPK/JNK pathway in type 2 diabetic mice and in MIN6 β-cells.Life Sci. 2014 Jun 27;107(1-2):42-9. doi: 10.1016/j.lfs.2014.04.034. Epub 2014 May 5. Life Sci. 2014. PMID: 24802125
-
The relevance of AMP-activated protein kinase in insulin-secreting β cells: a potential target for improving β cell function?J Physiol Biochem. 2019 Nov;75(4):423-432. doi: 10.1007/s13105-019-00706-3. Epub 2019 Nov 5. J Physiol Biochem. 2019. PMID: 31691163 Free PMC article. Review.
-
AMP-activated protein kinase in metabolic control and insulin signaling.Circ Res. 2007 Feb 16;100(3):328-41. doi: 10.1161/01.RES.0000256090.42690.05. Circ Res. 2007. PMID: 17307971 Review.
Cited by
-
Regulation of Pancreatic β Cell Mass by Cross-Interaction between CCAAT Enhancer Binding Protein β Induced by Endoplasmic Reticulum Stress and AMP-Activated Protein Kinase Activity.PLoS One. 2015 Jun 19;10(6):e0130757. doi: 10.1371/journal.pone.0130757. eCollection 2015. PLoS One. 2015. PMID: 26091000 Free PMC article.
-
AMP-activated protein kinase and pancreatic/duodenal homeobox-1 involved in insulin secretion under high leucine exposure in rat insulinoma beta-cells.J Cell Mol Med. 2009 Apr;13(4):758-70. doi: 10.1111/j.1582-4934.2009.00656.x. J Cell Mol Med. 2009. PMID: 19438972 Free PMC article.
-
AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes.J Biol Chem. 2010 Nov 26;285(48):37503-12. doi: 10.1074/jbc.M110.136796. Epub 2010 Sep 22. J Biol Chem. 2010. PMID: 20861022 Free PMC article.
-
Interaction between Autophagy and Senescence in Pancreatic Beta Cells.Biology (Basel). 2023 Sep 4;12(9):1205. doi: 10.3390/biology12091205. Biology (Basel). 2023. PMID: 37759604 Free PMC article. Review.
-
Evolution of β-Cell Replacement Therapy in Diabetes Mellitus: Islet Cell Transplantation.J Transplant. 2011;2011:247959. doi: 10.1155/2011/247959. Epub 2011 Oct 15. J Transplant. 2011. PMID: 22013505 Free PMC article.
References
-
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. - PubMed
-
- Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol. 2003;66:1433–1440. - PubMed
-
- Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol. 2004;4:259–268. - PubMed
-
- Pearl-Yafe M, Kaminitz A, Yolcu ES, Yaniv I, Stein J, Askenasy N. Pancreatic islets under attack: cellular and molecular effectors. Curr Pharm Des. 2007;13:749–760. - PubMed
-
- Lee SC, Pervaiz S. Apoptosis in the pathophysiology of diabetes mellitus. Int J Biochem Cell Biol. 2007;39:497–504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

