CD40 and CD80/86 act synergistically to regulate inflammation and mortality in polymicrobial sepsis
- PMID: 17989345
- PMCID: PMC2218847
- DOI: 10.1164/rccm.200703-515OC
CD40 and CD80/86 act synergistically to regulate inflammation and mortality in polymicrobial sepsis
Abstract
Rationale: Costimulatory molecules, including the CD40-CD154 and CD80/86-CD28 dyads, play a prominent role in regulating inflammation in the adaptive immune response. Studies from our group and others suggest a potentially important role for these costimulatory cascades in innate immunity as well.
Objectives: To determine the role of CD80/86 alone and in combination with CD40 in lethal polymicrobial sepsis in mice and humans.
Methods: The murine cecal ligation and puncture (CLP) model was used to determine the role of CD80/86 alone and in combination with CD40 using wild-type mice, CD80/86(-/-) mice, and novel CD40/80/86(-/-) mice. Expression of cell-bound and soluble costimulatory molecules was assessed in humans via ELISA and flow cytometry.
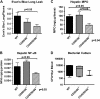
Measurements and main results: Lethal CLP was associated with up-regulation of CD40 and CD80/86 and their respective ligands CD28 and CD154 on innate effector cells. Blockade or deletion of CD80/86 attenuated mortality and inflammatory cytokine production during CLP. CD40/80/86(-/-) mice exhibited further reductions in mortality, lung injury, and inflammatory cytokine production compared with CD80/86(-/-) mice. Finally, humans with sepsis had increased monocyte expression of CD40 and CD80 compared with healthy control subjects; with higher levels in subjects requiring vasopressor support. Levels of soluble CD28 and CD154 were significantly higher in patients who died compared with those who lived.
Conclusions: These data demonstrate a central role for CD40 and CD80/86 in the innate immune response and suggest that combined inhibition of CD40 and CD80/86 may improve mortality in sepsis. Expression of costimulatory molecules may serve as biomarkers for outcome in septic patients.
Figures

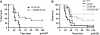
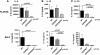
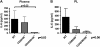

Similar articles
-
Differential role for CD80 and CD86 in the regulation of the innate immune response in murine polymicrobial sepsis.PLoS One. 2009 Aug 12;4(8):e6600. doi: 10.1371/journal.pone.0006600. PLoS One. 2009. PMID: 19672303 Free PMC article.
-
Sepsis-induced changes in macrophage co-stimulatory molecule expression: CD86 as a regulator of anti-inflammatory IL-10 response.Surg Infect (Larchmt). 2004 Winter;5(4):375-83. doi: 10.1089/sur.2004.5.375. Surg Infect (Larchmt). 2004. PMID: 15744129 Free PMC article.
-
Thymic medullary epithelium and thymocyte self-tolerance require cooperation between CD28-CD80/86 and CD40-CD40L costimulatory pathways.J Immunol. 2014 Jan 15;192(2):630-40. doi: 10.4049/jimmunol.1302550. Epub 2013 Dec 13. J Immunol. 2014. PMID: 24337745 Free PMC article.
-
CD28-CD80/86 and CD40-CD40L Interactions Promote Thymic Tolerance by Regulating Medullary Epithelial Cell and Thymocyte Development.Crit Rev Immunol. 2015;35(1):59-76. doi: 10.1615/critrevimmunol.2015012501. Crit Rev Immunol. 2015. PMID: 25746048 Free PMC article. Review.
-
T cells in the pathogenesis of systemic lupus erythematosus: potential roles of CD154-CD40 interactions and costimulatory molecules.Curr Rheumatol Rep. 2000 Feb;2(1):24-31. doi: 10.1007/s11926-996-0065-8. Curr Rheumatol Rep. 2000. PMID: 11123036 Review.
Cited by
-
Pneumonia in the first week after polytrauma is associated with reduced blood levels of soluble herpes virus entry mediator.Front Immunol. 2023 Dec 22;14:1259423. doi: 10.3389/fimmu.2023.1259423. eCollection 2023. Front Immunol. 2023. PMID: 38187375 Free PMC article.
-
Differential role for CD80 and CD86 in the regulation of the innate immune response in murine polymicrobial sepsis.PLoS One. 2009 Aug 12;4(8):e6600. doi: 10.1371/journal.pone.0006600. PLoS One. 2009. PMID: 19672303 Free PMC article.
-
Molecular and Clinical Characterization of CD80 Expression via Large-Scale Analysis in Breast Cancer.Front Pharmacol. 2022 Jun 22;13:869877. doi: 10.3389/fphar.2022.869877. eCollection 2022. Front Pharmacol. 2022. PMID: 35814211 Free PMC article.
-
Resolution of experimental lung injury by monocyte-derived inducible nitric oxide synthase.J Immunol. 2012 Sep 1;189(5):2234-45. doi: 10.4049/jimmunol.1102606. Epub 2012 Jul 27. J Immunol. 2012. PMID: 22844117 Free PMC article.
-
Increased CD4(+) T cell co-inhibitory immune receptor CEACAM1 in neonatal sepsis and soluble-CEACAM1 in meningococcal sepsis: a role in sepsis-associated immune suppression?PLoS One. 2013 Jul 22;8(7):e68294. doi: 10.1371/journal.pone.0068294. Print 2013. PLoS One. 2013. PMID: 23894299 Free PMC article.
References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–1310. - PubMed
-
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138–150. - PubMed
-
- Opal SM, Fisher CJ Jr, Dhainaut JF, Vincent JL, Brase R, Lowry SF, Sadoff JC, Slotman GJ, Levy H, Balk RA, et al.; Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. Crit Care Med 1997;25:1115–1124. - PubMed
-
- Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, Dal Nogare A, Nasraway S, Berman S, Cooney R, et al.; NORASEPT II Study Group. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. Lancet 1998;351:929–933. - PubMed
-
- Cohen J, Carlet J; International Sepsis Trial Study Group. INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-α in patients with sepsis. Crit Care Med 1996;24:1431–1440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

