Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis
- PMID: 17967865
- PMCID: PMC2223429
- DOI: 10.1128/MCB.01338-07
Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis
Abstract
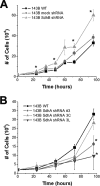
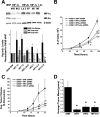
Mitochondrial complex II is a tumor suppressor comprised of four subunits (SdhA, SdhB, SdhC, and SdhD). Mutations in any of these should disrupt complex II enzymatic activity, yet defects in SdhA produce bioenergetic deficiency while defects in SdhB, SdhC, or SdhD induce tumor formation. The mechanisms underlying these differences are not known. We show that the inhibition of distal subunits of complex II, either pharmacologically or via RNA interference of SdhB, increases normoxic reactive oxygen species (ROS) production, increases hypoxia-inducible factor alpha (HIF-alpha) stabilization in an ROS-dependent manner, and increases growth rates in vitro and in vivo without affecting hypoxia-mediated activation of HIF-alpha. Proximal pharmacologic inhibition or RNA interference of complex II at SdhA, however, does not increase normoxic ROS production or HIF-alpha stabilization and results in decreased growth rates in vitro and in vivo. Furthermore, the enhanced growth rates resulting from SdhB suppression are inhibited by the suppression of HIF-1alpha and/or HIF-2alpha, indicating that the mechanism of SdhB-induced tumor formation relies upon ROS production and subsequent HIF-alpha activation. Therefore, differences in ROS production, HIF proliferation, and cell proliferation contribute to the differences in tumor phenotype in cells lacking SdhB as opposed to those lacking SdhA.
Figures
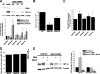
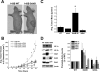
Similar articles
-
Loss of SDHB Elevates Catecholamine Synthesis and Secretion Depending on ROS Production and HIF Stabilization.Neurochem Res. 2016 Apr;41(4):696-706. doi: 10.1007/s11064-015-1738-3. Epub 2015 Nov 30. Neurochem Res. 2016. PMID: 26620190
-
Mitochondrial complex II regulates a distinct oxygen sensing mechanism in monocytes.Hum Mol Genet. 2017 Apr 1;26(7):1328-1339. doi: 10.1093/hmg/ddx041. Hum Mol Genet. 2017. PMID: 28204537 Free PMC article.
-
Identification of a signaling axis HIF-1α/microRNA-210/ISCU independent of SDH mutation that defines a subgroup of head and neck paragangliomas.J Clin Endocrinol Metab. 2012 Nov;97(11):E2194-200. doi: 10.1210/jc.2012-2410. Epub 2012 Sep 13. J Clin Endocrinol Metab. 2012. PMID: 22977270
-
Phenotypic dichotomy in mitochondrial complex II genetic disorders.J Mol Med (Berl). 2001 Sep;79(9):495-503. doi: 10.1007/s001090100267. J Mol Med (Berl). 2001. PMID: 11692162 Review.
-
Model animals for the study of oxidative stress from complex II.Biochim Biophys Acta. 2013 May;1827(5):588-97. doi: 10.1016/j.bbabio.2012.10.016. Epub 2012 Nov 6. Biochim Biophys Acta. 2013. PMID: 23142169 Review.
Cited by
-
The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling.Mol Cell. 2013 Jul 25;51(2):236-48. doi: 10.1016/j.molcel.2013.05.003. Epub 2013 Jun 6. Mol Cell. 2013. PMID: 23747014 Free PMC article.
-
TCA Cycle Defects and Cancer: When Metabolism Tunes Redox State.Int J Cell Biol. 2012;2012:161837. doi: 10.1155/2012/161837. Epub 2012 Jul 19. Int J Cell Biol. 2012. PMID: 22888353 Free PMC article.
-
Methylsulfonylmethane inhibits cortisol-induced stress through p53-mediated SDHA/HPRT1 expression in racehorse skeletal muscle cells: A primary step against exercise stress.Exp Ther Med. 2020 Jan;19(1):214-222. doi: 10.3892/etm.2019.8196. Epub 2019 Nov 13. Exp Ther Med. 2020. PMID: 31853292 Free PMC article.
-
SENP3 is responsible for HIF-1 transactivation under mild oxidative stress via p300 de-SUMOylation.EMBO J. 2009 Sep 16;28(18):2748-62. doi: 10.1038/emboj.2009.210. Epub 2009 Aug 13. EMBO J. 2009. PMID: 19680224 Free PMC article.
-
Dysregulation of Glucose Metabolism by Oncogenes and Tumor Suppressors in Cancer Cells.Asian Pac J Cancer Prev. 2018 Sep 26;19(9):2377-2390. doi: 10.22034/APJCP.2018.19.9.2377. Asian Pac J Cancer Prev. 2018. PMID: 30255690 Free PMC article. Review.
References
-
- Ackrell, B. A. C. 2000. Progress in understanding structure-function relationships in respiratory chain complex II. FEBS Lett. 4661-5. - PubMed
-
- Astrom, K., J. E. Cohen, J. E. Willett-Brozick, C. E. Aston, and B. E. Baysal. 2003. Altitude is a phenotypic modifier in hereditary paraganglioma type 1: evidence for an oxygen-sensing defect. Hum. Genet. 113228-237. - PubMed
-
- Baysal, B. E., R. E. Ferrell, J. E. Willett-Brozick, E. C. Lawrence, D. Myssiorek, A. Bosch, A. van der Mey, P. E. Taschner, W. S. Rubinstein, E. N. Myers, C. W. Richard III, C. J. Cornelisse, P. Devilee, and B. Devlin. 2000. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 287848-851. - PubMed
-
- Baysal, B. E., and E. N. Myers. 2002. Etiopathogenesis and clinical presentation of carotid body tumors. Microsc. Res. Tech. 59256-261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
