Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide
- PMID: 17962629
- PMCID: PMC3481783
- DOI: 10.1161/ATVBAHA.107.145466
Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide
Abstract
Objective: Microparticles of iron oxide (MPIO) distort magnetic field creating marked contrast effects far exceeding their physical size. We hypothesized that antibody-conjugated MPIO would enable magnetic resonance imaging (MRI) of endothelial cell adhesion molecules in mouse atherosclerosis.
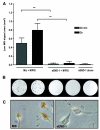
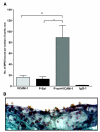
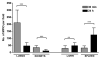
Methods and results: MPIO (4.5 microm) were conjugated to monoclonal antibodies against vascular cell adhesion molecule-1 (VCAM-MPIO) or P-selectin (P-selectin-MPIO). In vitro, VCAM-MPIO bound, in dose-dependent manner, to tumor necrosis factor (TNF)-alpha stimulated sEND-1 endothelial cells, as quantified by light microscopy (R2=0.94, P=0.03) and by MRI (R2=0.98, P=0.01). VCAM-MPIO binding was blocked by preincubation with soluble VCAM-1. To mimic leukocyte binding, MPIO targeting both VCAM-1 and P-selectin were administered in apolipoprotein E-/- mice. By light microscopy, dual-targeted MPIO binding to endothelium overlying aortic root atherosclerosis was 5- to 7-fold more than P-selectin-MPIO (P<0.05) or VCAM-MPIO (P<0.01) alone. Dual-targeted MPIO, injected intravenously in vivo bound aortic root endothelium and were quantifiable by MRI ex vivo (3.5-fold increase versus control; P<0.01). MPIO were well-tolerated in vivo, with sequestration in the spleen after 24 hours.
Conclusions: Dual-ligand MPIO bound to endothelium over atherosclerosis in vivo, under flow conditions. MPIO may provide a functional MRI probe for detecting endothelial-specific markers in a range of vascular pathologies.
Figures


Similar articles
-
A leukocyte-mimetic magnetic resonance imaging contrast agent homes rapidly to activated endothelium and tracks with atherosclerotic lesion macrophage content.Arterioscler Thromb Vasc Biol. 2012 Jun;32(6):1427-35. doi: 10.1161/ATVBAHA.111.241844. Epub 2012 Apr 12. Arterioscler Thromb Vasc Biol. 2012. PMID: 22499989 Free PMC article.
-
Anti-vascular cell adhesion molecule antibody M/K-2.7 and anti-P-selectin antibody RB40.34 conjugated microparticles of iron oxide.2008 Mar 8 [updated 2008 Apr 21]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Mar 8 [updated 2008 Apr 21]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641464 Free Books & Documents. Review.
-
Magnetic resonance imaging of brain inflammation using microparticles of iron oxide.Methods Mol Biol. 2011;680:103-15. doi: 10.1007/978-1-60761-901-7_7. Methods Mol Biol. 2011. PMID: 21153376
-
Imaging vulnerable plaques by targeting inflammation in atherosclerosis using fluorescent-labeled dual-ligand microparticles of iron oxide and magnetic resonance imaging.J Vasc Surg. 2018 May;67(5):1571-1583.e3. doi: 10.1016/j.jvs.2017.04.046. Epub 2017 Jun 22. J Vasc Surg. 2018. PMID: 28648478
-
Anti-vascular cell adhesion molecule antibody M/K-2.7–conjugated microparticles of iron oxide.2011 Jan 28 [updated 2011 Apr 14]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Jan 28 [updated 2011 Apr 14]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 21510041 Free Books & Documents. Review.
Cited by
-
MRI techniques for immunotherapy monitoring.J Immunother Cancer. 2022 Sep;10(9):e004708. doi: 10.1136/jitc-2022-004708. J Immunother Cancer. 2022. PMID: 36122963 Free PMC article. Review.
-
MRI Contrast Agents in Glycobiology.Molecules. 2022 Nov 28;27(23):8297. doi: 10.3390/molecules27238297. Molecules. 2022. PMID: 36500389 Free PMC article. Review.
-
Nanomedicine strategies for molecular targets with MRI and optical imaging.Future Med Chem. 2010 Mar;2(3):471-90. doi: 10.4155/fmc.10.5. Future Med Chem. 2010. PMID: 20485473 Free PMC article. Review.
-
Molecular imaging in cardiovascular disease: Which methods, which diseases?J Nucl Cardiol. 2013 Dec;20(6):990-1001. doi: 10.1007/s12350-013-9785-0. J Nucl Cardiol. 2013. PMID: 24092271 Free PMC article. Review.
-
MR and Targeted Molecular MRI of Vulnerable Plaques.Interv Neurol. 2013 Sep;1(3-4):124-31. doi: 10.1159/000346767. Interv Neurol. 2013. PMID: 25187773 Free PMC article. Review.
References
-
- Fayad ZA, Fuster V, Choudhury RP. CMR Atherothrombotic plaque imaging. In: Lardo AC, Fayad ZA, Chronos NA, Fuster V, editors. Cardiovascular Magnetic Resonance. Established and emerging applications. Martin Dunitz; London: 2003.
-
- Wood ML, Wehrli FW. Principles of magnetic resonance imaging. In: Stark DD, Bradley WG Jr, editors. Magnetic Resonance Imaging. Mosby; St Louis: 1999.
-
- Choudhury RP, Fuster V, Badimon JJ, Fisher EA, Fayad ZA. MRI and characterization of atherosclerotic plaque: emerging applications and molecular imaging. Arterioscler Thromb Vasc Biol. 2002;22:1065–1074. Abstract/FREE Full Text. - PubMed
-
- Choudhury RP, Fuster V, Fayad ZA. Molecular, cellular and functional imaging of atherothrombosis. Nat Rev Drug Discov. 2004;3:913–925. CrossRefMedline. - PubMed
-
- Lipinski MJ, Fuster V, Fisher EA, Fayad ZA. Technology insight: targeting of biological molecules for evaluation of high-risk atherosclerotic plaques with magnetic resonance imaging. Nat Clin Pract Cardiovasc Med. 2004;1:48–55. Medline. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

