Confinement or the nature of the interface? Dynamics of nanoscopic water
- PMID: 17958424
- PMCID: PMC2532509
- DOI: 10.1021/ja073977d
Confinement or the nature of the interface? Dynamics of nanoscopic water
Abstract
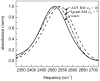
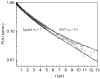
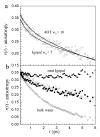
The dynamics of water confined in two different types of reverse micelles are studied using ultrafast infrared pump-probe spectroscopy of the hydroxyl OD stretch of HOD in H2O. Reverse micelles of the surfactant Aerosol-OT (ionic head group) in isooctane and the surfactant Igepal CO 520 (nonionic head group) in 50/50 wt % cyclohexane/hexane are prepared to have the same diameter water nanopools. Measurements of the IR spectra and vibrational lifetimes show that the identity of the surfactant head groups affects the local environment experienced by the water molecules inside the reverse micelles. The orientational dynamics (time-dependent anisotropy), which is a measure of the hydrogen bond network rearrangement, are very similar for the confined water in the two types of reverse micelles. The results demonstrate that confinement by an interface to form a nanoscopic water pool is a primary factor governing the dynamics of nanoscopic water rather than the presence of charged groups at the interface.
Figures
Similar articles
-
Water dynamics--the effects of ions and nanoconfinement.J Phys Chem B. 2008 May 1;112(17):5279-90. doi: 10.1021/jp7121856. Epub 2008 Mar 28. J Phys Chem B. 2008. PMID: 18370431
-
Water dynamics in small reverse micelles in two solvents: two-dimensional infrared vibrational echoes with two-dimensional background subtraction.J Chem Phys. 2011 Feb 7;134(5):054512. doi: 10.1063/1.3532542. J Chem Phys. 2011. PMID: 21303143 Free PMC article.
-
Dynamics of water at the interface in reverse micelles: measurements of spectral diffusion with two-dimensional infrared vibrational echoes.J Phys Chem B. 2011 Oct 13;115(40):11658-70. doi: 10.1021/jp206903k. Epub 2011 Sep 22. J Phys Chem B. 2011. PMID: 21899355
-
Ultrafast energy transfer in water-AOT reverse micelles.J Phys Chem B. 2007 Dec 27;111(51):14193-207. doi: 10.1021/jp0723158. Epub 2007 Nov 30. J Phys Chem B. 2007. PMID: 18047308
-
Analysis of water in confined geometries and at interfaces.Annu Rev Anal Chem (Palo Alto Calif). 2010;3:89-107. doi: 10.1146/annurev-anchem-070109-103410. Annu Rev Anal Chem (Palo Alto Calif). 2010. PMID: 20636035 Review.
Cited by
-
Radical re-appraisal of water structure in hydrophilic confinement.Chem Phys Lett. 2013 Dec 18;590:1-15. doi: 10.1016/j.cplett.2013.10.075. Chem Phys Lett. 2013. PMID: 25843963 Free PMC article.
-
Vibrational sum-frequency generation spectroscopy at the water/lipid interface: molecular dynamics simulation study.J Am Chem Soc. 2010 May 12;132(18):6434-42. doi: 10.1021/ja100508n. J Am Chem Soc. 2010. PMID: 20394423 Free PMC article.
-
The Journey of 1-Keto-1,2,3,4-Tetrahydrocarbazole Based Fluorophores: From Inception to Implementation.J Fluoresc. 2022 Nov;32(6):2023-2052. doi: 10.1007/s10895-022-03004-2. Epub 2022 Jul 13. J Fluoresc. 2022. PMID: 35829843 Review.
-
Reverse micelles as a tool for probing solvent modulation of protein dynamics: Reverse micelle encapsulated hemoglobin.Chem Phys. 2013 Aug 30;430:88-97. doi: 10.1016/j.chemphys.2013.04.006. Chem Phys. 2013. PMID: 24039330 Free PMC article.
-
An ion's perspective on the molecular motions of nanoconfined water: a two-dimensional infrared spectroscopy study.J Phys Chem B. 2013 Aug 22;117(33):9775-84. doi: 10.1021/jp406725a. Epub 2013 Aug 8. J Phys Chem B. 2013. PMID: 23855349 Free PMC article.
References
-
- Liu L, Faraone A, Mou CY, Yen CW, Chen SH. J Phys: Condens Matter. 2004;16:S5403–S5436.
-
- Ramsay JDF, Poinsignon C. Langmuir. 1987;3:320–326.
-
- Floquet N, Coulomb JP, Dufau N, Andre G, Kahn R. Physica B. 2004;350:265–269.
-
- Mitra S, Mukhopadhyay R, Tsukushi I, Ikeda S. J Phys: Condens Matter. 2001;13:8455–8465.
-
- Bellisent-Funel MC, Chen SH, Zanotti JM. Phys Rev E. 1995;51:4558–4569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

