Positron emission tomography (PET) imaging of neuroblastoma and melanoma with 64Cu-SarAr immunoconjugates
- PMID: 17954911
- PMCID: PMC2077283
- DOI: 10.1073/pnas.0708436104
Positron emission tomography (PET) imaging of neuroblastoma and melanoma with 64Cu-SarAr immunoconjugates
Abstract
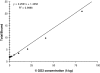
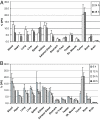
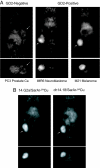
The advancement of positron emission tomography (PET) depends on the development of new radiotracers that will complement (18)F-FDG. Copper-64 ((64)Cu) is a promising PET radionuclide, particularly for antibody-targeted imaging, but the high in vivo lability of conventional chelates has limited its clinical application. The objective of this work was to evaluate the novel chelating agent SarAr (1-N-(4-aminobenzyl)-3, 6,10,13,16,19-hexaazabicyclo[6.6.6] eicosane-1,8-diamine) for use in developing a new class of tumor-specific (64)Cu radiopharmaceuticals for imaging neuroblastoma and melanoma. The anti-GD2 monoclonal antibody (mAb) 14.G2a, and its chimeric derivative, ch14.18, target disialogangliosides that are overexpressed on neuroblastoma and melanoma. Both mAbs were conjugated to SarAr using carbodiimide coupling. Radiolabeling with (64)Cu resulted in >95% of the (64)Cu being chelated by the immunoconjugate. Specific activities of at least 10 microCi/microg (1 Ci = 37 GBq) were routinely achieved, and no additional purification was required after (64)Cu labeling. Solid-phase radioimmunoassays and intact cell-binding assays confirmed retention of bioactivity. Biodistribution studies in athymic nude mice bearing s.c. neuroblastoma (IMR-6, NMB-7) and melanoma (M21) xenografts showed that 15-20% of the injected dose per gram accumulated in the tumor at 24 hours after injection, and only 5-10% of the injected dose accumulated in the liver, a lower value than typically seen with other chelators. Uptake by a GD2-negative tumor xenograft was significantly lower (<5% injected dose per gram). MicroPET imaging confirmed significant uptake of the tracer in GD-2-positive tumors, with minimal uptake in GD-2-negative tumors and nontarget tissues such as liver. The (64)Cu-SarAr-mAb system described here is potentially applicable to (64)Cu-PET imaging with a broad range of antibody or peptide-based imaging agents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Imaging cancer using PET--the effect of the bifunctional chelator on the biodistribution of a (64)Cu-labeled antibody.Nucl Med Biol. 2011 Jan;38(1):29-38. doi: 10.1016/j.nucmedbio.2010.07.003. Epub 2010 Oct 27. Nucl Med Biol. 2011. PMID: 21220127 Free PMC article.
-
In vitro and in vivo evaluation of 64Cu-labeled SarAr-bombesin analogs in gastrin-releasing peptide receptor-expressing prostate cancer.J Nucl Med. 2011 Mar;52(3):470-7. doi: 10.2967/jnumed.110.082826. Epub 2011 Feb 14. J Nucl Med. 2011. PMID: 21321264 Free PMC article.
-
The ionic charge of copper-64 complexes conjugated to an engineered antibody affects biodistribution.Bioconjug Chem. 2015 Apr 15;26(4):707-17. doi: 10.1021/acs.bioconjchem.5b00049. Epub 2015 Mar 12. Bioconjug Chem. 2015. PMID: 25719414
-
[64Cu](1-N-(4-aminobenzyl)-3,6,10,13,16,19-hexaazabicyclo[6.6.6]-eicosane-1,8-diamine)-anti-GD2 monoclonal antibody.In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641203 Free Books & Documents. Review.
-
Sarar technology for the application of Copper-64 in biology and materials science.Q J Nucl Med Mol Imaging. 2008 Jun;52(2):193-202. Epub 2008 Jan 5. Q J Nucl Med Mol Imaging. 2008. PMID: 18174877 Review.
Cited by
-
Image guided biodistribution and pharmacokinetic studies of theranostics.Theranostics. 2012;2(11):1040-53. doi: 10.7150/thno.4652. Epub 2012 Nov 5. Theranostics. 2012. PMID: 23227121 Free PMC article. Review.
-
Trackable and Targeted Phage as Positron Emission Tomography (PET) Agent for Cancer Imaging.Theranostics. 2011;1:371-80. doi: 10.7150/thno/v01p0371. Epub 2011 Nov 18. Theranostics. 2011. PMID: 22211143 Free PMC article.
-
Copper-67-Labeled Bombesin Peptide for Targeted Radionuclide Therapy of Prostate Cancer.Pharmaceuticals (Basel). 2022 Jun 8;15(6):728. doi: 10.3390/ph15060728. Pharmaceuticals (Basel). 2022. PMID: 35745647 Free PMC article.
-
Imaging cancer using PET--the effect of the bifunctional chelator on the biodistribution of a (64)Cu-labeled antibody.Nucl Med Biol. 2011 Jan;38(1):29-38. doi: 10.1016/j.nucmedbio.2010.07.003. Epub 2010 Oct 27. Nucl Med Biol. 2011. PMID: 21220127 Free PMC article.
-
In vitro and in vivo analysis of [(64)Cu-NO2A-8-Aoc-BBN(7-14)NH(2)]: a site-directed radiopharmaceutical for positron-emission tomography imaging of T-47D human breast cancer tumors.Nucl Med Biol. 2009 Feb;36(2):171-81. doi: 10.1016/j.nucmedbio.2008.11.005. Nucl Med Biol. 2009. PMID: 19217529 Free PMC article.
References
-
- Rohren EM, Turkington TG, Coleman RE. Radiology. 2004;231:305–332. - PubMed
-
- Gambhir SS. Nat Rev Cancer. 2002;2:683–693. - PubMed
-
- Jerusalem G, Hustinx R, Beguin Y, Fillet G. Eur J Cancer. 2003;39:1525–1534. - PubMed
-
- Wilkinson MD, Fulham MJ. Clin Nuclear Med. 2003;28:780–781. - PubMed
-
- Smith SV. J Inorg Biochem. 2004;98:1874–1901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

