Accumulation of substrates of the anaphase-promoting complex (APC) during human cytomegalovirus infection is associated with the phosphorylation of Cdh1 and the dissociation and relocalization of APC subunits
- PMID: 17942546
- PMCID: PMC2224378
- DOI: 10.1128/JVI.02010-07
Accumulation of substrates of the anaphase-promoting complex (APC) during human cytomegalovirus infection is associated with the phosphorylation of Cdh1 and the dissociation and relocalization of APC subunits
Abstract
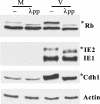
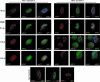
Cell cycle dysregulation upon human cytomegalovirus (HCMV) infection of human fibroblasts is associated with the inactivation of the anaphase-promoting complex (APC), a multisubunit E3 ubiquitin ligase, and accumulation of its substrates. Here, we have further elucidated the mechanism(s) by which HCMV-induced inactivation of the APC occurs. Our results show that Cdh1 accumulates in a phosphorylated form that may prevent its association with and activation of the APC. The accumulation of Cdh1, but not its phosphorylation, appears to be cyclin-dependent kinase dependent. The lack of an association of exogenously added Cdh1 with the APC from infected cells indicates that the core APC also may be impaired. This is further supported by an examination of the localization and composition of the APC. Coimmunoprecipitation studies show that both Cdh1 and the subunit APC1 become dissociated from the complex. In addition, immunofluorescence analysis demonstrates that as the infection progresses, several subunits redistribute to the cytoplasm, while APC1 remains nuclear. Dissociation of the core complex itself would account for not only the observed inactivity but also its inability to bind to Cdh1. Taken together, these results illustrate that HCMV has adopted multiple mechanisms to inactivate the APC, which underscores its importance for a productive infection.
Figures

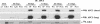


Similar articles
-
Inactivation and disassembly of the anaphase-promoting complex during human cytomegalovirus infection is associated with degradation of the APC5 and APC4 subunits and does not require UL97-mediated phosphorylation of Cdh1.J Virol. 2010 Oct;84(20):10832-43. doi: 10.1128/JVI.01260-10. Epub 2010 Aug 4. J Virol. 2010. PMID: 20686030 Free PMC article.
-
Studies on the Contribution of Human Cytomegalovirus UL21a and UL97 to Viral Growth and Inactivation of the Anaphase-Promoting Complex/Cyclosome (APC/C) E3 Ubiquitin Ligase Reveal a Unique Cellular Mechanism for Downmodulation of the APC/C Subunits APC1, APC4, and APC5.J Virol. 2015 Jul;89(13):6928-39. doi: 10.1128/JVI.00403-15. Epub 2015 Apr 22. J Virol. 2015. PMID: 25903336 Free PMC article.
-
Human cytomegalovirus inactivates the G0/G1-APC/C ubiquitin ligase by Cdh1 dissociation.Cell Cycle. 2005 Oct;4(10):1435-9. doi: 10.4161/cc.4.10.2077. Epub 2005 Oct 2. Cell Cycle. 2005. PMID: 16138013
-
Non-mitotic functions of the Anaphase-Promoting Complex.Semin Cell Dev Biol. 2011 Aug;22(6):572-8. doi: 10.1016/j.semcdb.2011.03.010. Epub 2011 Mar 23. Semin Cell Dev Biol. 2011. PMID: 21439391 Review.
-
Thinking within the D box: initial identification of Cdh1-APC substrates in the nervous system.Mol Cell Neurosci. 2007 Mar;34(3):281-7. doi: 10.1016/j.mcn.2006.11.019. Epub 2007 Jan 12. Mol Cell Neurosci. 2007. PMID: 17223572 Review.
Cited by
-
Control the host cell cycle: viral regulation of the anaphase-promoting complex.J Virol. 2013 Aug;87(16):8818-25. doi: 10.1128/JVI.00088-13. Epub 2013 Jun 12. J Virol. 2013. PMID: 23760246 Free PMC article. Review.
-
Proteasome subunits relocalize during human cytomegalovirus infection, and proteasome activity is necessary for efficient viral gene transcription.J Virol. 2010 Mar;84(6):3079-93. doi: 10.1128/JVI.02236-09. Epub 2009 Dec 30. J Virol. 2010. PMID: 20042513 Free PMC article.
-
Cdk5 phosphorylates Cdh1 and modulates cyclin B1 stability in excitotoxicity.EMBO J. 2008 Oct 22;27(20):2736-45. doi: 10.1038/emboj.2008.195. Epub 2008 Sep 25. EMBO J. 2008. PMID: 18818692 Free PMC article.
-
Cell cycle-independent expression of immediate-early gene 3 results in G1 and G2 arrest in murine cytomegalovirus-infected cells.J Virol. 2008 Oct;82(20):10188-98. doi: 10.1128/JVI.01212-08. Epub 2008 Jul 30. J Virol. 2008. PMID: 18667506 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus Lytic Replication Is Independent of Anaphase-Promoting Complex Activity.J Virol. 2020 Jun 16;94(13):e02079-19. doi: 10.1128/JVI.02079-19. Print 2020 Jun 16. J Virol. 2020. PMID: 32295923 Free PMC article.
References
-
- Bresnahan, W. A., I. Boldogh, E. A. Thompson, and T. Albrecht. 1996. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology 224156-160. - PubMed
-
- Bresnahan, W. A., E. A. Thompson, and T. Albrecht. 1997. Human cytomegalovirus infection results in altered Cdk2 subcellular localization. J. Gen. Virol. 781993-1997. - PubMed
-
- Castro, A., C. Bernis, S. Vigneron, J. C. Labbe, and T. Lorca. 2005. The anaphase-promoting complex: a key factor in the regulation of cell cycle. Oncogene 24314-325. - PubMed
-
- De Azevedo, W. F., S. Leclerc, L. Meijer, L. Havlicek, M. Strnad, and S. H. Kim. 1997. Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. Eur. J. Biochem. 243518-526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

