Human cytomegalovirus specifically controls the levels of the endoplasmic reticulum chaperone BiP/GRP78, which is required for virion assembly
- PMID: 17942541
- PMCID: PMC2224369
- DOI: 10.1128/JVI.01881-07
Human cytomegalovirus specifically controls the levels of the endoplasmic reticulum chaperone BiP/GRP78, which is required for virion assembly
Abstract
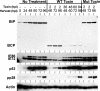
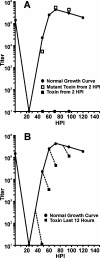
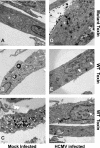
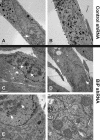
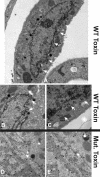
The endoplasmic reticulum (ER) chaperone BiP/GRP78 regulates ER function and the unfolded protein response (UPR). Human cytomegalovirus infection of human fibroblasts induces the UPR but modifies it to benefit viral replication. BiP/GRP78 protein levels are tightly regulated during infection, rising after 36 h postinfection (hpi), peaking at 60 hpi, and decreasing thereafter. To determine the effects of this regulation on viral replication, BiP/GRP78 was depleted using the SubAB subtilase cytotoxin, which rapidly and specifically cleaves BiP/GRP78. Toxin treatment of infected cells for 12-h periods beginning at 36, 48, 60, and 84 hpi caused complete loss of BiP but had little effect on viral protein synthesis. However, progeny virion formation was significantly inhibited, suggesting that BiP/GRP78 is important for virion formation. Electron microscopic analysis showed that infected cells were resistant to the toxin and showed none of the cytotoxic effects seen in uninfected cells. However, all viral activity in the cytoplasm ceased, with nucleocapsids remaining in the nucleus or concentrated in the cytoplasmic space just outside of the outer nuclear membrane. These data suggest that one effect of the controlled expression of BiP/GRP78 in infected cells is to aid in cytoplasmic virion assembly and egress.
Figures

Similar articles
-
The endoplasmic reticulum chaperone BiP/GRP78 is important in the structure and function of the human cytomegalovirus assembly compartment.J Virol. 2009 Nov;83(22):11421-8. doi: 10.1128/JVI.00762-09. Epub 2009 Sep 9. J Virol. 2009. PMID: 19741001 Free PMC article.
-
Role of the endoplasmic reticulum chaperone BiP, SUN domain proteins, and dynein in altering nuclear morphology during human cytomegalovirus infection.J Virol. 2010 Jul;84(14):7005-17. doi: 10.1128/JVI.00719-10. Epub 2010 May 19. J Virol. 2010. PMID: 20484513 Free PMC article.
-
Enterovirus 71 induces dsRNA/PKR-dependent cytoplasmic redistribution of GRP78/BiP to promote viral replication.Emerg Microbes Infect. 2016 Mar 23;5(3):e23. doi: 10.1038/emi.2016.20. Emerg Microbes Infect. 2016. PMID: 27004760 Free PMC article.
-
GRP78 in lung cancer.J Transl Med. 2021 Mar 21;19(1):118. doi: 10.1186/s12967-021-02786-6. J Transl Med. 2021. PMID: 33743739 Free PMC article. Review.
-
The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends.J Biol Chem. 2019 Feb 8;294(6):2098-2108. doi: 10.1074/jbc.REV118.002804. Epub 2018 Dec 18. J Biol Chem. 2019. PMID: 30563838 Free PMC article. Review.
Cited by
-
Structure, biological functions and applications of the AB5 toxins.Trends Biochem Sci. 2010 Jul;35(7):411-8. doi: 10.1016/j.tibs.2010.02.003. Epub 2010 Mar 2. Trends Biochem Sci. 2010. PMID: 20202851 Free PMC article. Review.
-
The endoplasmic reticulum chaperone BiP/GRP78 is important in the structure and function of the human cytomegalovirus assembly compartment.J Virol. 2009 Nov;83(22):11421-8. doi: 10.1128/JVI.00762-09. Epub 2009 Sep 9. J Virol. 2009. PMID: 19741001 Free PMC article.
-
Triggering unfolded protein response by 2-Deoxy-D-glucose inhibits porcine epidemic diarrhea virus propagation.Antiviral Res. 2014 Jun;106:33-41. doi: 10.1016/j.antiviral.2014.03.007. Epub 2014 Mar 25. Antiviral Res. 2014. PMID: 24681123 Free PMC article.
-
Fatty acid elongase 7 catalyzes lipidome remodeling essential for human cytomegalovirus replication.Cell Rep. 2015 Mar 3;10(8):1375-85. doi: 10.1016/j.celrep.2015.02.003. Epub 2015 Feb 26. Cell Rep. 2015. PMID: 25732827 Free PMC article.
-
Proteostasis in Viral Infection: Unfolding the Complex Virus-Chaperone Interplay.Cold Spring Harb Perspect Biol. 2020 Mar 2;12(3):a034090. doi: 10.1101/cshperspect.a034090. Cold Spring Harb Perspect Biol. 2020. PMID: 30858229 Free PMC article. Review.
References
-
- Arsham, A. M., J. J. Howell, and M. C. Simon. 2003. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J. Biol. Chem. 27829655-29660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

