Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells
- PMID: 17938236
- PMCID: PMC2118472
- DOI: 10.1084/jem.20071401
Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells
Abstract
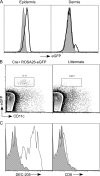
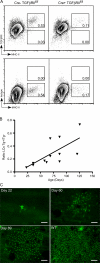
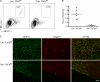
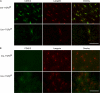
Langerhans cells (LCs) are bone marrow (BM)-derived epidermal dendritic cells (DCs) that develop from precursors found in the dermis. Epidermal LCs are absent in transforming growth factor (TGF) beta1-deficient mice. It is not clear whether TGFbeta1 acts directly on LC precursors to promote maturation or whether it acts on accessory cells, which in turn affect LC precursors. In addition, the physiologic source of TGFbeta1 is uncertain because BM chimera experiments showed that neither hematopoietic nor nonhematopoietic-derived TGFbeta1 is required for LC development. To address these issues, we created mice transgenic for a bacterial artificial chromosome (BAC) containing the gene for human Langerin into which Cre recombinase had been inserted by homologous recombination (Langerin-Cre). These mice express Cre selectively in LCs, and they were bred to floxed TGFbetaRII and TGFbeta1 mice, thereby generating mice with LCs that either cannot respond to or generate TGFbeta1, respectively. Langerin-Cre TGFbetaRII mice had substantially reduced numbers of epidermal LCs, demonstrating that TGFbeta1 acts directly on LCs in vivo. Interestingly, Langerin-Cre TGFbeta1 mice also had very few LCs both in the steady state and after BM transplantation. Thus, TGFbeta1 derived from LCs acts directly on LCs through an autocrine/paracrine loop, and it is required for LC development and/or survival.
Figures

Similar articles
-
The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells.J Exp Med. 2007 Dec 24;204(13):3119-31. doi: 10.1084/jem.20071724. Epub 2007 Dec 17. J Exp Med. 2007. PMID: 18086861 Free PMC article.
-
Conditional deletion of TGF-βR1 using Langerin-Cre mice results in Langerhans cell deficiency and reduced contact hypersensitivity.J Immunol. 2011 Nov 15;187(10):5069-76. doi: 10.4049/jimmunol.1101880. Epub 2011 Oct 12. J Immunol. 2011. PMID: 21998450
-
Autocrine/paracrine TGF-β1 inhibits Langerhans cell migration.Proc Natl Acad Sci U S A. 2012 Jun 26;109(26):10492-7. doi: 10.1073/pnas.1119178109. Epub 2012 Jun 11. Proc Natl Acad Sci U S A. 2012. PMID: 22689996 Free PMC article.
-
Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin.Immunol Rev. 2010 Mar;234(1):120-41. doi: 10.1111/j.0105-2896.2009.00886.x. Immunol Rev. 2010. PMID: 20193016 Free PMC article. Review.
-
Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells.Nat Rev Immunol. 2008 Dec;8(12):935-47. doi: 10.1038/nri2455. Nat Rev Immunol. 2008. PMID: 19029989 Review.
Cited by
-
Specialized role of migratory dendritic cells in peripheral tolerance induction.J Clin Invest. 2013 Feb;123(2):844-54. doi: 10.1172/JCI65260. Epub 2013 Jan 9. J Clin Invest. 2013. PMID: 23298832 Free PMC article.
-
In vivo function of Langerhans cells and dermal dendritic cells.Trends Immunol. 2010 Dec;31(12):446-51. doi: 10.1016/j.it.2010.08.006. Epub 2010 Oct 28. Trends Immunol. 2010. PMID: 21035396 Free PMC article. Review.
-
Langerhans cells suppress contact hypersensitivity responses via cognate CD4 interaction and langerhans cell-derived IL-10.J Immunol. 2009 Oct 15;183(8):5085-93. doi: 10.4049/jimmunol.0901884. J Immunol. 2009. PMID: 19801524 Free PMC article.
-
Tumor-promoting role of TGFβ1 signaling in ultraviolet B-induced skin carcinogenesis is associated with cutaneous inflammation and lymph node migration of dermal dendritic cells.Carcinogenesis. 2014 Apr;35(4):959-66. doi: 10.1093/carcin/bgt486. Epub 2013 Dec 20. Carcinogenesis. 2014. PMID: 24363069 Free PMC article.
-
Identification and characterization of murine glycoprotein 2-expressing intestinal dendritic cells.Scand J Immunol. 2022 Nov;96(5):e13219. doi: 10.1111/sji.13219. Epub 2022 Oct 17. Scand J Immunol. 2022. PMID: 37807915 Free PMC article.
References
-
- Romani, N., S. Holzmann, C.H. Tripp, F. Koch, and P. Stoitzner. 2003. Langerhans cells - dendritic cells of the epidermis. APMIS. 111:725–740. - PubMed
-
- Hemmi, H., M. Yoshino, H. Yamazaki, M. Naito, T. Iyoda, Y. Omatsu, S. Shimoyama, J.J. Letterio, T. Nakabayashi, H. Tagaya, et al. 2001. Skin antigens in the steady state are trafficked to regional lymph nodes by transforming growth factor-beta1-dependent cells. Int. Immunol. 13:695–704. - PubMed
-
- Kripke, M.L., C.G. Munn, A. Jeevan, J.M. Tang, and C. Bucana. 1990. Evidence that cutaneous antigen-presenting cells migrate to regional lymph nodes during contact sensitization. J. Immunol. 145:2833–2838. - PubMed
-
- Larregina, A.T., A.E. Morelli, L.A. Spencer, A.J. Logar, S.C. Watkins, A.W. Thomson, and L.D. Falo Jr. 2001. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat. Immunol. 2:1151–1158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

