Lengthening the G(1) phase of neural progenitor cells is concurrent with an increase of symmetric neuron generating division after stroke
- PMID: 17928800
- PMCID: PMC2749512
- DOI: 10.1038/sj.jcbfm.9600556
Lengthening the G(1) phase of neural progenitor cells is concurrent with an increase of symmetric neuron generating division after stroke
Abstract
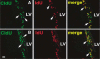
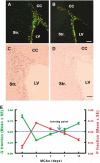
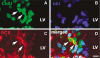
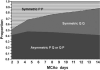
The proportion of neural progenitors that remain in (P fraction) and exit from (Q fraction) the cell cycle determines the degree of neurogenesis. Using S-phase labeling with 5-bromo-2'-deoxyuridine and a double nucleoside analog-labeling scheme, we measured the cell-cycle kinetics of neural progenitors and estimated the proportion of P and Q fractions in the subventricular zone (SVZ) of adult rats subjected to stroke. Stroke increased SVZ cell proliferation, starting 2 days, reaching a maximum 4 and 7 days after stroke. The cell-cycle length (T(C)) of SVZ cells changed dynamically over a period of 2 to 14 days after stroke, with the shortest length of 11 h at 2 days after stroke. The reduction of the T(C) resulted from a decrease of the G(1) phase because the G(2), M, and S phases were unchanged. In addition, during this period, reduction of the G(1) phase was concomitant with an increase in the P fraction, whereas an augmentation of the Q fraction was associated with lengthening of the G(1) phase. Furthermore, approximately 90% of cells that exited the cell cycle were neurons and the population of a pair of dividing daughter cells with a neuronal marker increased from 9% at 2 days to 26% at 14 days after stroke. These data suggest that stroke triggers early expansion of the progenitor pool via shortening the cell-cycle length and retaining daughter cells within the cell cycle, and the lengthening of G(1) leads to daughter cells exiting the cell cycle and differentiating into neurons.
Figures

Similar articles
-
Reduction of the cell cycle length by decreasing G1 phase and cell cycle reentry expand neuronal progenitor cells in the subventricular zone of adult rat after stroke.J Cereb Blood Flow Metab. 2006 Jun;26(6):857-63. doi: 10.1038/sj.jcbfm.9600237. J Cereb Blood Flow Metab. 2006. PMID: 16251885
-
Stroke transiently increases subventricular zone cell division from asymmetric to symmetric and increases neuronal differentiation in the adult rat.J Neurosci. 2004 Jun 23;24(25):5810-5. doi: 10.1523/JNEUROSCI.1109-04.2004. J Neurosci. 2004. PMID: 15215303 Free PMC article.
-
The linkage of neural progenitor cell cycle profiles between embryonic and adult stroke models: Analytical approach II.J Neurosci Methods. 2008 Jan 30;167(2):376-83. doi: 10.1016/j.jneumeth.2007.08.015. Epub 2007 Aug 25. J Neurosci Methods. 2008. PMID: 17928064 Free PMC article.
-
Gene profiles within the adult subventricular zone niche: proliferation, differentiation and migration of neural progenitor cells in the ischemic brain.Curr Mol Med. 2007 Aug;7(5):459-62. doi: 10.2174/156652407781387136. Curr Mol Med. 2007. PMID: 17691960 Review.
-
Making bigger brains-the evolution of neural-progenitor-cell division.J Cell Sci. 2008 Sep 1;121(Pt 17):2783-93. doi: 10.1242/jcs.023465. J Cell Sci. 2008. PMID: 18716282 Review.
Cited by
-
Function of neural stem cells in ischemic brain repair processes.J Cereb Blood Flow Metab. 2016 Dec;36(12):2034-2043. doi: 10.1177/0271678X16674487. Epub 2016 Oct 14. J Cereb Blood Flow Metab. 2016. PMID: 27742890 Free PMC article. Review.
-
Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain.J Neurosci. 2009 Apr 8;29(14):4408-19. doi: 10.1523/JNEUROSCI.6003-08.2009. J Neurosci. 2009. PMID: 19357268 Free PMC article.
-
In Vivo Targeted Magnetic Resonance Imaging of Endogenous Neural Stem Cells in the Adult Rodent Brain.Biomed Res Int. 2015;2015:131054. doi: 10.1155/2015/131054. Epub 2015 Oct 25. Biomed Res Int. 2015. PMID: 26583085 Free PMC article.
-
Stem cells in cerebrovascular diseases.J Mol Neurosci. 2013 Oct;51(2):262-4. doi: 10.1007/s12031-013-0009-5. Epub 2013 Apr 5. J Mol Neurosci. 2013. PMID: 23559314 No abstract available.
-
The subependymal zone neurogenic niche: a beating heart in the centre of the brain: how plastic is adult neurogenesis? Opportunities for therapy and questions to be addressed.Brain. 2009 Nov;132(Pt 11):2909-21. doi: 10.1093/brain/awp237. Epub 2009 Sep 22. Brain. 2009. PMID: 19773354 Free PMC article. Review.
References
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70. - PubMed
-
- Aten JA, Stap J, Hoebe R, Bakker PJ. Application and detection of IdUrd and CldUrd as two independent cell-cycle markers. Methods Cell Biol. 1994;41:317–26. - PubMed
-
- Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–42. - PubMed
-
- Caviness VS, Jr, Takahashi T, Nowakowski RS. Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 1995;18:379–83. - PubMed
Publication types
MeSH terms
Grants and funding
- P01 NS042345-05/NS/NINDS NIH HHS/United States
- P01 NS042345-050003/NS/NINDS NIH HHS/United States
- R01 NS038292/NS/NINDS NIH HHS/United States
- P50 NS023393-19A10001/NS/NINDS NIH HHS/United States
- R01 HL064766/HL/NHLBI NIH HHS/United States
- R01 NS 38292/NS/NINDS NIH HHS/United States
- P01 NS042345/NS/NINDS NIH HHS/United States
- P01 NS 23393/NS/NINDS NIH HHS/United States
- R01 HL 64766/HL/NHLBI NIH HHS/United States
- P50 NS023393-19A1/NS/NINDS NIH HHS/United States
- P01 NS 42345/NS/NINDS NIH HHS/United States
- P01 NS023393/NS/NINDS NIH HHS/United States
- P50 NS023393/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

