The BMP ligand Gbb gates the expression of synaptic homeostasis independent of synaptic growth control
- PMID: 17920019
- PMCID: PMC2699048
- DOI: 10.1016/j.neuron.2007.08.006
The BMP ligand Gbb gates the expression of synaptic homeostasis independent of synaptic growth control
Abstract
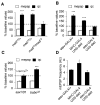
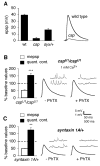
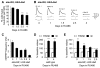
Inhibition of postsynaptic glutamate receptors at the Drosophila NMJ initiates a compensatory increase in presynaptic release termed synaptic homeostasis. BMP signaling is necessary for normal synaptic growth and stability. It remains unknown whether BMPs have a specific role during synaptic homeostasis and, if so, whether BMP signaling functions as an instructive retrograde signal that directly modulates presynaptic transmitter release. Here, we demonstrate that the BMP receptor (Wit) and ligand (Gbb) are necessary for the rapid induction of synaptic homeostasis. We also provide evidence that both Wit and Gbb have functions during synaptic homeostasis that are separable from NMJ growth. However, further genetic experiments demonstrate that Gbb does not function as an instructive retrograde signal during synaptic homeostasis. Rather, our data indicate that Wit and Gbb function via the downstream transcription factor Mad and that Mad-mediated signaling is continuously required during development to confer competence of motoneurons to express synaptic homeostasis.
Figures
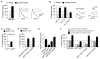
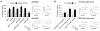

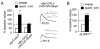
Similar articles
-
The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction.Neuron. 2003 Jul 17;39(2):241-54. doi: 10.1016/s0896-6273(03)00426-4. Neuron. 2003. PMID: 12873382
-
Retrograde BMP signaling at the synapse: a permissive signal for synapse maturation and activity-dependent plasticity.J Neurosci. 2013 Nov 6;33(45):17937-50. doi: 10.1523/JNEUROSCI.6075-11.2013. J Neurosci. 2013. PMID: 24198381 Free PMC article.
-
Crimpy inhibits the BMP homolog Gbb in motoneurons to enable proper growth control at the Drosophila neuromuscular junction.Development. 2011 Aug;138(15):3273-86. doi: 10.1242/dev.066142. Development. 2011. PMID: 21750037 Free PMC article.
-
Orchestrating development and function: retrograde BMP signaling in the Drosophila nervous system.Trends Neurosci. 2004 Mar;27(3):143-7. doi: 10.1016/j.tins.2004.01.004. Trends Neurosci. 2004. PMID: 15036879 Review.
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
Cited by
-
Rapid active zone remodeling during synaptic plasticity.J Neurosci. 2011 Apr 20;31(16):6041-52. doi: 10.1523/JNEUROSCI.6698-10.2011. J Neurosci. 2011. PMID: 21508229 Free PMC article.
-
DBL-1, a TGF-β, is essential for Caenorhabditis elegans aversive olfactory learning.Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):17081-6. doi: 10.1073/pnas.1205982109. Epub 2012 Sep 26. Proc Natl Acad Sci U S A. 2012. PMID: 23019581 Free PMC article.
-
Importin-beta11 regulates synaptic phosphorylated mothers against decapentaplegic, and thereby influences synaptic development and function at the Drosophila neuromuscular junction.J Neurosci. 2010 Apr 14;30(15):5253-68. doi: 10.1523/JNEUROSCI.3739-09.2010. J Neurosci. 2010. PMID: 20392948 Free PMC article.
-
Roles for sleep in memory: insights from the fly.Curr Opin Neurobiol. 2019 Feb;54:120-126. doi: 10.1016/j.conb.2018.10.006. Epub 2018 Oct 23. Curr Opin Neurobiol. 2019. PMID: 30366270 Free PMC article. Review.
-
The less things change, the more they are different: contributions of long-term synaptic plasticity and homeostasis to memory.Learn Mem. 2014 Feb 14;21(3):128-34. doi: 10.1101/lm.027326.112. Learn Mem. 2014. PMID: 24532836 Free PMC article. Review.
References
-
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, Goodman CS. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. - PubMed
-
- Allan DW, St Pierre SE, Miguel-Aliaga I, Thor S. Specification of neuropeptide cell identity by the integration of retrograde BMP signaling and a combinatorial transcription factor code. Cell. 2003;113:73–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

