Stromal fibroblasts in colorectal liver metastases originate from resident fibroblasts and generate an inflammatory microenvironment
- PMID: 17916596
- PMCID: PMC2043521
- DOI: 10.2353/ajpath.2007.060661
Stromal fibroblasts in colorectal liver metastases originate from resident fibroblasts and generate an inflammatory microenvironment
Abstract
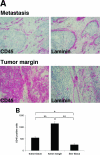
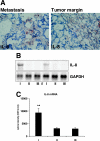
Cancer-associated stromal fibroblasts (CAFs) are the main cellular constituents of reactive stroma in primary and metastatic cancer. We analyzed phenotypical characteristics of CAFs from human colorectal liver metastases (CLMs) and their role in inflammation and cancer progression. CAFs displayed a vimentin(+), alpha-smooth-muscle actin(+), and Thy-1(+) phenotype similar to resident portal-located liver fibroblasts (LFs). We demonstrated that CLMs are inflammatory sites showing stromal expression of interleukin-8 (IL-8), a chemokine related to invasion and angiogenesis. In vitro analyses revealed a striking induction of IL-8 expression in CAFs and LFs by tumor necrosis factor-alpha (TNF-alpha). The effect of TNF-alpha on CAFs is inhibited by the nuclear factor-kappaB inhibitor parthenolide. Conditioned medium of CAFs and LFs similarly stimulated the migration of DLD-1, Colo-678, HuH7 carcinoma cells, and human umbilical vein endothelial cells in vitro. Pretreatment of CAFs with TNF-alpha increased the chemotaxis of Colo-678 colon carcinoma cells by conditioned medium of CAFs; however, blockage of IL-8 activity showed no inhibitory effect. In conclusion, these data raise the possibility that the majority of CAFs in CLM originate from resident LFs. TNF-alpha-induced up-regulation of IL-8 via nuclear factor-kappaB in CAFs is an inflammatory pathway, potentially permissive for cancer invasion that may represent a novel therapeutic target.
Figures


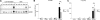

Similar articles
-
TNF-alpha similarly induces IL-6 and MCP-1 in fibroblasts from colorectal liver metastases and normal liver fibroblasts.Biochem Biophys Res Commun. 2010 Jul 2;397(3):586-91. doi: 10.1016/j.bbrc.2010.05.163. Biochem Biophys Res Commun. 2010. PMID: 20617559
-
Regulation of the inflammatory profile of stromal cells in human breast cancer: prominent roles for TNF-α and the NF-κB pathway.Stem Cell Res Ther. 2015 May 1;6(1):87. doi: 10.1186/s13287-015-0080-7. Stem Cell Res Ther. 2015. PMID: 25928089 Free PMC article.
-
Transforming growth factor-1 promotes the transcriptional activation of plasminogen activator inhibitor type 1 in carcinoma-associated fibroblasts.Mol Med Rep. 2012 Nov;6(5):1001-5. doi: 10.3892/mmr.2012.1020. Epub 2012 Aug 7. Mol Med Rep. 2012. PMID: 22895748
-
Regulation of heterogeneous cancer-associated fibroblasts: the molecular pathology of activated signaling pathways.J Exp Clin Cancer Res. 2020 Jun 16;39(1):112. doi: 10.1186/s13046-020-01611-0. J Exp Clin Cancer Res. 2020. PMID: 32546182 Free PMC article. Review.
-
Stromal cells in tumor microenvironment and breast cancer.Cancer Metastasis Rev. 2013 Jun;32(1-2):303-15. doi: 10.1007/s10555-012-9415-3. Cancer Metastasis Rev. 2013. PMID: 23114846 Free PMC article. Review.
Cited by
-
The Roles of Mesenchymal Stromal/Stem Cells in Tumor Microenvironment Associated with Inflammation.Mediators Inflamm. 2016;2016:7314016. doi: 10.1155/2016/7314016. Epub 2016 Aug 18. Mediators Inflamm. 2016. PMID: 27630452 Free PMC article. Review.
-
Tumor stroma as targets for cancer therapy.Pharmacol Ther. 2013 Feb;137(2):200-15. doi: 10.1016/j.pharmthera.2012.10.003. Epub 2012 Oct 12. Pharmacol Ther. 2013. PMID: 23064233 Free PMC article. Review.
-
Role of pancreatic stellate cells in pancreatic cancer metastasis.Am J Pathol. 2010 Nov;177(5):2585-96. doi: 10.2353/ajpath.2010.090899. Epub 2010 Oct 7. Am J Pathol. 2010. PMID: 20934972 Free PMC article.
-
Differentiation and transdifferentiation potentials of cancer stem cells.Oncotarget. 2015 Nov 24;6(37):39550-63. doi: 10.18632/oncotarget.6098. Oncotarget. 2015. PMID: 26474460 Free PMC article. Review.
-
Unveiling the role of tumor reactive stroma in cholangiocarcinoma: an opportunity for new therapeutic strategies.Transl Gastrointest Cancer. 2013 Jul;2(3):130-144. doi: 10.3978/j.issn.2224-4778.2013.04.02. Transl Gastrointest Cancer. 2013. PMID: 28989865 Free PMC article.
References
-
- Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. - PubMed
-
- Khatri VP, Petrelli NJ, Belghiti J. Extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit? J Clin Oncol. 2005;23:8490–8499. - PubMed
-
- Desmoulière A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–517. - PubMed
-
- Mazzocca A, Coppari R, De Franco R, Cho JY, Libermann TA, Pinzani M, Toker A. A secreted form of ADAM9 promotes carcinoma invasion through tumor-stromal interactions. Cancer Res. 2005;65:4728–4738. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

