Anorexia induced by activation of serotonin 5-HT4 receptors is mediated by increases in CART in the nucleus accumbens
- PMID: 17913892
- PMCID: PMC2042207
- DOI: 10.1073/pnas.0701471104
Anorexia induced by activation of serotonin 5-HT4 receptors is mediated by increases in CART in the nucleus accumbens
Abstract
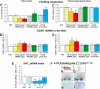
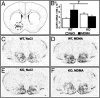
Anorexia nervosa is a growing concern in mental health, often inducing death. The potential neuronal deficits that may underlie abnormal inhibitions of food intake, however, remain largely unexplored. We hypothesized that anorexia may involve altered signaling events within the nucleus accumbens (NAc), a brain structure involved in reward. We show here that direct stimulation of serotonin (5-hydroxytryptamine, 5-HT) 4 receptors (5-HT(4)R) in the NAc reduces the physiological drive to eat and increases CART (cocaine- and amphetamine-regulated transcript) mRNA levels in fed and food-deprived mice. It further shows that injecting 5-HT(4)R antagonist or siRNA-mediated 5-HT(4)R knockdown into the NAc induced hyperphagia only in fed mice. This hyperphagia was not associated with changes in CART mRNA expression in the NAc in fed and food-deprived mice. Results include that 5-HT(4)R control CART mRNA expression into the NAc via a cAMP/PKA signaling pathway. Considering that CART may interfere with food- and drug-related rewards, we tested whether the appetite suppressant properties of 3,4-N-methylenedioxymethamphetamine (MDMA, ecstasy) involve the 5-HT(4)R. Using 5-HT(4)R knockout mice, we demonstrate that 5-HT(4)R are required for the anorectic effect of MDMA as well as for the MDMA-induced enhancement of CART mRNA expression in the NAc. Directly injecting CART peptide or CART siRNA into the NAc reduces or increases food consumption, respectively. Finally, stimulating 5-HT(4)R- and MDMA-induced anorexia were both reduced by injecting CART siRNA into the NAc. Collectively, these results demonstrate that 5-HT(4)R-mediated up-regulation of CART in the NAc triggers the appetite-suppressant effects of ecstasy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
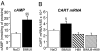

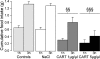

Similar articles
-
The nucleus accumbens 5-HTR₄-CART pathway ties anorexia to hyperactivity.Transl Psychiatry. 2012 Dec 11;2(12):e203. doi: 10.1038/tp.2012.131. Transl Psychiatry. 2012. PMID: 23233022 Free PMC article.
-
Serotonin 5-HT4 receptors in the nucleus accumbens are specifically involved in the appetite suppressant and not locomotor stimulant effects of MDMA ('ecstasy').Psychopharmacology (Berl). 2011 Feb;213(2-3):355-63. doi: 10.1007/s00213-010-1982-9. Epub 2010 Aug 26. Psychopharmacology (Berl). 2011. PMID: 20740276
-
[Feeding disorders in 5-HT4 receptor knockout mice].J Soc Biol. 2004;198(1):37-49. J Soc Biol. 2004. PMID: 15146954 French.
-
The effects of methylenedioxymethamphetamine (MDMA, "Ecstasy") on monoaminergic neurotransmission in the central nervous system.Prog Neurobiol. 1996 Aug;49(5):455-79. doi: 10.1016/0301-0082(96)00027-5. Prog Neurobiol. 1996. PMID: 8895996 Review.
-
Actions of 3,4-methylenedioxymethamphetamine (MDMA) on cerebral dopaminergic, serotonergic and cholinergic neurons.Pharmacol Biochem Behav. 2008 Aug;90(2):198-207. doi: 10.1016/j.pbb.2007.10.003. Epub 2007 Oct 16. Pharmacol Biochem Behav. 2008. PMID: 18035407 Free PMC article. Review.
Cited by
-
Leptin reverses declines in satiation in weight-reduced obese humans.Am J Clin Nutr. 2012 Feb;95(2):309-17. doi: 10.3945/ajcn.111.012385. Epub 2012 Jan 11. Am J Clin Nutr. 2012. PMID: 22237063 Free PMC article. Clinical Trial.
-
DNIC-mediated analgesia produced by a supramaximal electrical or a high-dose formalin conditioning stimulus: roles of opioid and alpha2-adrenergic receptors.J Biomed Sci. 2010 Mar 19;17(1):19. doi: 10.1186/1423-0127-17-19. J Biomed Sci. 2010. PMID: 20302612 Free PMC article.
-
The serotonergic system dysfunction in diabetes mellitus.Front Cell Neurosci. 2022 Jul 14;16:899069. doi: 10.3389/fncel.2022.899069. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35910256 Free PMC article. Review.
-
Updated Review of the Toxicity of Selected Fusarium Toxins and Their Modified Forms.Toxins (Basel). 2021 Oct 29;13(11):768. doi: 10.3390/toxins13110768. Toxins (Basel). 2021. PMID: 34822552 Free PMC article. Review.
-
Pharmacological manipulations in animal models of anorexia and binge eating in relation to humans.Br J Pharmacol. 2014 Oct;171(20):4767-84. doi: 10.1111/bph.12789. Epub 2014 Sep 5. Br J Pharmacol. 2014. PMID: 24866852 Free PMC article. Review.
References
-
- Sullivan PF. Am J Psychiatry. 1995;152:1073–1074. - PubMed
-
- Signorini A, De Filippo E, Panico S, De Caprio C, Pasanisi F, Contaldo F. Eur J Clin Nutr. 2007;61:119–122. - PubMed
-
- Compan V. In: Serotonin Receptors in Neurobiology. Chattopadhyay A, editor. Boca Raton, FL: RC; 2007. pp. 157–180.
-
- Vickers SP, Clifton PG, Dourish CT, Tecott LH. Psychopharmacology. 1999;143:309–314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

