Novel decapeptides that bind avidly and deliver radioisotope to colon cancer cells
- PMID: 17912343
- PMCID: PMC1978517
- DOI: 10.1371/journal.pone.0000964
Novel decapeptides that bind avidly and deliver radioisotope to colon cancer cells
Abstract
Background: The rapidly growing field of targeted tumor therapy often utilizes an antibody, sometimes tagged with a tumor-ablating material such as radioisotope, directed against a specific molecule.
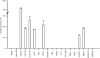
Methodology/principal findings: This report describes the discovery of nine novel decapeptides which can be radioactively labeled, bind to, and deliver (32)P to colon cancer cells. The decapeptides vary from one another by one to three amino acids and demonstrate vastly different binding abilities. The most avidly binding decapeptide can permanently deliver very high levels of radioisotope to the adenocarcinoma cancer cell lines at an efficiency 35 to 150 times greater than to a variety of other cell types, including cell lines derived from other types of cancer or from normal tissue.
Conclusions/significance: This experimental approach represents a new example of a strategy, termed peptide binding therapy, for the potential treatment of colorectal and other adenocarcinomas.
Conflict of interest statement
Figures

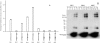

Similar articles
-
Generation of small 32P-labeled peptides as a potential approach to colorectal cancer therapy.PLoS One. 2008 Jun 25;3(6):e2508. doi: 10.1371/journal.pone.0002508. PLoS One. 2008. PMID: 18575578 Free PMC article.
-
Pharmaceutical approaches to colon targeted drug delivery systems.J Pharm Pharm Sci. 2003 Jan-Apr;6(1):33-66. J Pharm Pharm Sci. 2003. PMID: 12753729 Review.
-
A New NT4 Peptide-Based Drug Delivery System for Cancer Treatment.Molecules. 2020 Feb 28;25(5):1088. doi: 10.3390/molecules25051088. Molecules. 2020. PMID: 32121130 Free PMC article.
-
Oral 5-fluorouracil colon-specific delivery through in vivo pellet coating for colon cancer and aberrant crypt foci treatment.Int J Pharm. 2014 Jul 1;468(1-2):178-86. doi: 10.1016/j.ijpharm.2014.04.006. Epub 2014 Apr 5. Int J Pharm. 2014. PMID: 24709212
-
Ligand-based vascular targeting of disease.ChemMedChem. 2007 Jan;2(1):22-40. doi: 10.1002/cmdc.200600181. ChemMedChem. 2007. PMID: 17154429 Review.
Cited by
-
Generation of small 32P-labeled peptides as a potential approach to colorectal cancer therapy.PLoS One. 2008 Jun 25;3(6):e2508. doi: 10.1371/journal.pone.0002508. PLoS One. 2008. PMID: 18575578 Free PMC article.
-
Comprehensive biostatistical analysis of CpG island methylator phenotype in colorectal cancer using a large population-based sample.PLoS One. 2008;3(11):e3698. doi: 10.1371/journal.pone.0003698. Epub 2008 Nov 12. PLoS One. 2008. PMID: 19002263 Free PMC article.
References
-
- Edwards BK, Brown ML, Wingo PA, Howe HL, Ward F, et al. Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J. Natl. Cancer Inst. 2005;97:1407–1427. - PubMed
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, et al. Cancer Statistics, 2007. CA Cancer J. Clin. 2007;57:43–66. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, et al. Use of Chemotherapy plus a monoclonal antibody against HER2 for metastastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001;344:783–792. - PubMed
-
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2 positive breast cancer. N. Engl. J. Med. 2005;353:1673–1684. - PubMed
-
- Tan-Chiu E, Yothers G, Romond E, Geyer CE, Ewer M, et al. Assessment of cardiac dysfunstion in a randomized trial comparing doxorubicin and cyclophosphamide followed by placlitaxel, with or without Trastuzumab as adjuvant therapy in node positive, human epidermal growth factor receptor 2-overexpressing breast cancer. J.Clin. Oncol. 2005;23:7811–7819. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

