Targeted disruption of the glutaredoxin 1 gene does not sensitize adult mice to tissue injury induced by ischemia/reperfusion and hyperoxia
- PMID: 17893043
- PMCID: PMC2196211
- DOI: 10.1016/j.freeradbiomed.2007.07.025
Targeted disruption of the glutaredoxin 1 gene does not sensitize adult mice to tissue injury induced by ischemia/reperfusion and hyperoxia
Abstract
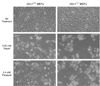
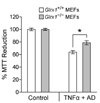
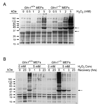
To understand the physiological function of glutaredoxin, a thiotransferase catalyzing the reduction of mixed disulfides of protein and glutathione, we generated a line of knockout mice deficient in the cytosolic glutaredoxin 1 (Grx1). To our surprise, mice deficient in Grx1 were not more susceptible to acute oxidative insults in models of heart and lung injury induced by ischemia/reperfusion and hyperoxia, respectively, suggesting that either changes in S-glutathionylation status of cytosolic proteins are not the major cause of such tissue injury or developmental adaptation in the Glrx1-knockout animals alters the response to oxidative insult. In contrast, mouse embryonic fibroblasts (MEFs) isolated from Grx1-deficient mice displayed an increased vulnerability to diquat and paraquat, but they were not more susceptible to cell death induced by hydrogen peroxide (H(2)O(2)) and diamide. A deficiency in Grx1 also sensitized MEFs to protein S-glutathionylation in response to H(2)O(2) treatment and retarded deglutathionylation of the S-glutathionylated proteins, especially for a single prominent protein band. Additional experiments showed that MEFs lacking Grx1 were more tolerant to apoptosis induced by tumor necrosis factor alphaplus actinomycin D. These findings suggest that various oxidants may damage the cells via distinct mechanisms in which the action of Grx1 may or may not be protective and Grx1 may exert its function on specific target proteins.
Figures





Similar articles
-
Ablation of glutaredoxin 1 promotes pulmonary angiogenesis and alveolar formation in hyperoxia-injured lungs by modifying HIF-1α stability and inhibiting the NF-κB pathway.Biochem Biophys Res Commun. 2020 Apr 30;525(2):528-535. doi: 10.1016/j.bbrc.2020.02.129. Epub 2020 Feb 26. Biochem Biophys Res Commun. 2020. PMID: 32113683
-
Glutaredoxin 1 (Grx1) Protects Human Retinal Pigment Epithelial Cells From Oxidative Damage by Preventing AKT Glutathionylation.Invest Ophthalmol Vis Sci. 2015 May;56(5):2821-32. doi: 10.1167/iovs.14-15876. Invest Ophthalmol Vis Sci. 2015. PMID: 25788646
-
Glutaredoxin 1 protects dopaminergic cells by increased protein glutathionylation in experimental Parkinson's disease.Antioxid Redox Signal. 2012 Dec 15;17(12):1676-93. doi: 10.1089/ars.2011.4474. Epub 2012 Sep 14. Antioxid Redox Signal. 2012. PMID: 22816731 Free PMC article.
-
The nature of antioxidant defense mechanisms: a lesson from transgenic studies.Environ Health Perspect. 1998 Oct;106 Suppl 5(Suppl 5):1219-28. doi: 10.1289/ehp.98106s51219. Environ Health Perspect. 1998. PMID: 9788901 Free PMC article. Review.
-
Critical Roles of Glutaredoxin in Brain Cells-Implications for Parkinson's Disease.Antioxid Redox Signal. 2019 Apr 1;30(10):1352-1368. doi: 10.1089/ars.2017.7411. Epub 2018 Jan 5. Antioxid Redox Signal. 2019. PMID: 29183158 Free PMC article. Review.
Cited by
-
Ablation of Glutaredoxin-1 Modulates House Dust Mite-Induced Allergic Airways Disease in Mice.Am J Respir Cell Mol Biol. 2016 Sep;55(3):377-86. doi: 10.1165/rcmb.2015-0401OC. Am J Respir Cell Mol Biol. 2016. PMID: 27035878 Free PMC article.
-
S-Denitrosylation: A Crosstalk between Glutathione and Redoxin Systems.Antioxidants (Basel). 2022 Sep 28;11(10):1921. doi: 10.3390/antiox11101921. Antioxidants (Basel). 2022. PMID: 36290644 Free PMC article. Review.
-
Glutathione adducts induced by ischemia and deletion of glutaredoxin-1 stabilize HIF-1α and improve limb revascularization.Proc Natl Acad Sci U S A. 2016 May 24;113(21):6011-6. doi: 10.1073/pnas.1524198113. Epub 2016 May 9. Proc Natl Acad Sci U S A. 2016. PMID: 27162359 Free PMC article.
-
Regulation of cell physiology and pathology by protein S-glutathionylation: lessons learned from the cardiovascular system.Antioxid Redox Signal. 2012 Mar 15;16(6):524-42. doi: 10.1089/ars.2011.4336. Antioxid Redox Signal. 2012. PMID: 22010840 Free PMC article. Review.
-
Redox amplification of apoptosis by caspase-dependent cleavage of glutaredoxin 1 and S-glutathionylation of Fas.J Cell Biol. 2009 Jan 26;184(2):241-52. doi: 10.1083/jcb.200807019. J Cell Biol. 2009. PMID: 19171757 Free PMC article.
References
-
- Freeman B, Crapo JD. Biology of disease: free radicals and tissue injury. Lab. Invest. 1982;47:412–426. - PubMed
-
- Finkel T. Redox-dependent signal transduction. FEBS Lett. 2000;476:52–54. - PubMed
-
- Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: role in reversible protein S-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid. Redox Signal. 2005;7:348–366. - PubMed
-
- Rhee SG. Cell signaling: H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. - PubMed
-
- Forman HJ, Fisher AB. Antioxidant defense. In: Gilbert DL, editor. Oxygen and Living Processes: An Interdisciplinary Approach. New York: Springer-Verlag; 1982. pp. 235–249.
Publication types
MeSH terms
Substances
Grants and funding
- P30 ES006639-13/ES/NIEHS NIH HHS/United States
- AG024413/AG/NIA NIH HHS/United States
- P01 AG015885/AG/NIA NIH HHS/United States
- HL87271/HL/NHLBI NIH HHS/United States
- R15 HL087271-01/HL/NHLBI NIH HHS/United States
- R15 HL087271/HL/NHLBI NIH HHS/United States
- HL63317/HL/NHLBI NIH HHS/United States
- P30 ES06639/ES/NIEHS NIH HHS/United States
- R01 HL063317-01/HL/NHLBI NIH HHS/United States
- P30 ES006639/ES/NIEHS NIH HHS/United States
- R01 AG024413-03/AG/NIA NIH HHS/United States
- AG15885/AG/NIA NIH HHS/United States
- P01 AG015885-06/AG/NIA NIH HHS/United States
- R01 AG024413/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

