Administration of a CD25-directed immunotoxin, LMB-2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivo
- PMID: 17878392
- PMCID: PMC2134981
- DOI: 10.4049/jimmunol.179.7.4919
Administration of a CD25-directed immunotoxin, LMB-2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivo
Abstract
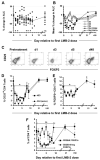
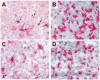
CD25+ CD4+ T regulatory (Treg) cells regulate peripheral self tolerance and possess the ability to suppress antitumor responses, which may in part explain the poor clinical response of cancer patients undergoing active immunization protocols. We have previously shown that in vitro incubation of human PBMC with LMB-2, a CD25-directed immunotoxin, significantly reduced CD25+ FOXP3+ CD4+ Treg cells without impairing the function of the remaining lymphocytes. In the current study, eight patients with metastatic melanoma were treated with LMB-2 followed by MART-1 and gp100-specific peptide vaccination. LMB-2 administration resulted in a preferential, transient reduction in mean circulating CD25+ CD4+ T cell number, from 83 +/- 16 cells/microl to a nadir of 17 +/- 5 cells/microl, a 79.1% reduction. FOXP3 analysis revealed a less robust depletion with mean FOXP3+ CD4+ Treg cell number decreasing from 74 +/- 15 cells/microl to 36 +/- 8 cells/microl, a 51.4% reduction. FOXP3+ CD4+ Treg cells that survived LMB-2-mediated cytotoxicity expressed little or no CD25. Similar to the peripheral blood, immunohistochemical analysis showed a 68.9% mean reduction in FOXP3+ CD4+ Treg cell frequency in evaluable lesions. Despite inducing a reduction in Treg cell numbers in vivo, LMB-2 therapy did not augment the immune response to cancer vaccination and no patient experienced an objective response or autoimmunity. These data demonstrate the capacity of a CD25-directed immunotoxin to selectively mediate a transient partial reduction in circulating and tumor-infiltrating Treg cells in vivo, and suggest that more comprehensive Treg cell elimination may be required to bolster antitumor responses in patients with metastatic melanoma.
Figures
Similar articles
-
Partial reduction of human FOXP3+ CD4 T cells in vivo after CD25-directed recombinant immunotoxin administration.J Immunother. 2008 Feb-Mar;31(2):189-98. doi: 10.1097/CJI.0b013e31815dc0e8. J Immunother. 2008. PMID: 18481388 Free PMC article. Clinical Trial.
-
Selective elimination of human regulatory T lymphocytes in vitro with the recombinant immunotoxin LMB-2.J Immunother. 2006 Mar-Apr;29(2):208-14. doi: 10.1097/01.cji.0000187959.45803.0c. J Immunother. 2006. PMID: 16531821 Free PMC article.
-
Inability to mediate prolonged reduction of regulatory T Cells after transfer of autologous CD25-depleted PBMC and interleukin-2 after lymphodepleting chemotherapy.J Immunother. 2007 May-Jun;30(4):438-47. doi: 10.1097/CJI.0b013e3180600ff9. J Immunother. 2007. PMID: 17457218 Free PMC article.
-
Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells.Ann N Y Acad Sci. 2009 Sep;1174:99-106. doi: 10.1111/j.1749-6632.2009.04939.x. Ann N Y Acad Sci. 2009. PMID: 19769742 Review.
-
TCR peptide vaccination in multiple sclerosis: boosting a deficient natural regulatory network that may involve TCR-specific CD4+CD25+ Treg cells.Curr Drug Targets Inflamm Allergy. 2005 Apr;4(2):217-29. doi: 10.2174/1568010053586327. Curr Drug Targets Inflamm Allergy. 2005. PMID: 15853744 Review.
Cited by
-
Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication.Immunol Rev. 2011 May;241(1):104-18. doi: 10.1111/j.1600-065X.2011.01007.x. Immunol Rev. 2011. PMID: 21488893 Free PMC article. Review.
-
Regulatory (FOXP3+) T cells as target for immune therapy of cervical intraepithelial neoplasia and cervical cancer.Cancer Sci. 2009 Jun;100(6):1112-7. doi: 10.1111/j.1349-7006.2009.01153.x. Cancer Sci. 2009. PMID: 19514119 Free PMC article.
-
Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human melanoma.Cancer Immun. 2009 Apr 2;9:3. Cancer Immun. 2009. PMID: 19338264 Free PMC article. Review.
-
Impact of anti-CD25 monoclonal antibody on dendritic cell-tumor fusion vaccine efficacy in a murine melanoma model.J Transl Med. 2013 Jun 17;11:148. doi: 10.1186/1479-5876-11-148. J Transl Med. 2013. PMID: 23768240 Free PMC article.
-
Cognitive disorders in mice: cytokine signaling pathways as therapeutic targets.OMICS. 2012 Jan-Feb;16(1-2):71-7. doi: 10.1089/omi.2011.0037. OMICS. 2012. PMID: 22321016 Free PMC article.
References
-
- Lee KH, Wang E, Nielsen MB, Wunderlich J, Migueles S, Connors M, Steinberg SM, Rosenberg SA, Marincola FM. Increased vaccine-specific T cell frequency after peptide-based vaccination correlates with increased susceptibility to in vitro stimulation but does not lead to tumor regression. J Immunol. 1999;163:6292– 6300. - PubMed
-
- Nielsen MB, Monsurro V, Migueles SA, Wang E, Perez-Diez A, Lee KH, Kammula U, Rosenberg SA, Marincola FM. Status of activation of circulating vaccine-elicited CD8+ T cells. J Immunol. 2000;165:2287–2296. - PubMed
-
- Pittet MJ, Valmori D, Dunbar PR, Speiser DE, Lienard D, Lejeune F, Fleischhauer K, Cerundolo V, Cerottini JC, Romero P. High frequencies of naive Melan-A/MART-1-specific CD8+ T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J Exp Med. 1999;190:705–715. - PMC - PubMed
-
- Speiser DE, Lienard D, Pittet MJ, Batard P, Rimoldi D, Guillaume P, Cerottini JC, Romero P. In vivo activation of melanoma-specific CD8+ T cells by endogenous tumor antigen and peptide vaccines. A comparison to virus-specific T cells. Eur J Immunol. 2002;32:731–741. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

