Listeriolysin O is an improved protein carrier for lymphoma immunoglobulin idiotype and provides systemic protection against 38C13 lymphoma
- PMID: 17876582
- PMCID: PMC11030947
- DOI: 10.1007/s00262-007-0388-y
Listeriolysin O is an improved protein carrier for lymphoma immunoglobulin idiotype and provides systemic protection against 38C13 lymphoma
Abstract
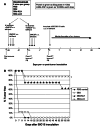
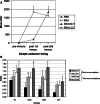
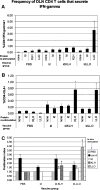
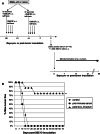
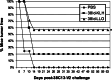
Follicular lymphoma (FL) is a disease that responds to current treatment regimens; however, patients in general relapse with increasingly refractory disease. Idiotype-based vaccines are currently under trial for the treatment of FL. These vaccines comprise the patient's BCR idiotype (Id) as the tumor antigen conjugated to the protein carrier Keyhole Limpet Hemocyanin (KLH); however, other protein carriers may enhance the immune response to the lymphoma Id. In this study we investigated whether an alternate carrier, Listeriolysin O (LLO), would amplify the immune response to Id protein and provide better protection against challenge by 38C13 murine lymphoma. The Id-LLO vaccine compared favorably against Id-KLH in tumor-protection studies and both vaccines provided systemic immunity against 38C13 lymphoma. However, the immune response to the two conjugates was different in that Id-LLO induced a more powerful Th1 response characterized by high titer IgG2a anti-Id antibodies after one immunization and the presence of CD4 cells secreting IFN-gamma. In vivo studies demonstrated that immune serum contributed to the anti-lymphoma efficacy seen following Id-LLO immunization. Interestingly, Id-LLO immunized mice, when challenged twice with 38C13 lymphoma provided better protection against challenge by the BCR loss variant 38C13-V2, suggesting that Id-LLO immunized mice have more potential to develop epitope spreading than Id-KLH. In conclusion, Id-LLO compared favorably against Id-KLH in its anti-lymphoma efficacy. Furthermore, Id-LLO induced a more potent humoral and cell-mediated immune response and promoted epitope spreading after lymphoma challenge. Thus, anti-Id vaccines incorporating LLO may be a better therapeutic option for treatment of B-cell lymphoma.
Figures

Similar articles
-
Sulfhydryl-based tumor antigen-carrier protein conjugates stimulate superior antitumor immunity against B cell lymphomas.J Immunol. 2008 Sep 15;181(6):4131-40. doi: 10.4049/jimmunol.181.6.4131. J Immunol. 2008. PMID: 18768870
-
Idiotype vaccination post-bone marrow transplantation for B-cell lymphoma: initial studies in a murine model.Cancer Detect Prev. 1991;15(4):323-5. Cancer Detect Prev. 1991. PMID: 1794139
-
Vaccination with syngeneic, lymphoma-derived immunoglobulin idiotype combined with granulocyte/macrophage colony-stimulating factor primes mice for a protective T-cell response.Proc Natl Acad Sci U S A. 1996 Oct 1;93(20):10972-7. doi: 10.1073/pnas.93.20.10972. Proc Natl Acad Sci U S A. 1996. PMID: 8855293 Free PMC article.
-
Idiotypic vaccination for B-cell malignancies as a model for therapeutic cancer vaccines: from prototype protein to second generation vaccines.Haematologica. 2002 Sep;87(9):989-1001. Haematologica. 2002. PMID: 12217812 Review.
-
Translational development of vaccination strategies in follicular NHL.Best Pract Res Clin Haematol. 2011 Jun;24(2):295-304. doi: 10.1016/j.beha.2011.03.007. Epub 2011 May 6. Best Pract Res Clin Haematol. 2011. PMID: 21658625 Review.
Cited by
-
Lm-LLO-Based Immunotherapies and HPV-Associated Disease.J Oncol. 2012;2012:542851. doi: 10.1155/2012/542851. Epub 2012 Feb 2. J Oncol. 2012. PMID: 22481930 Free PMC article.
-
Recombinant AAV-CEA Tumor Vaccine in Combination with an Immune Adjuvant Breaks Tolerance and Provides Protective Immunity.Mol Ther Oncolytics. 2018 Dec 13;12:41-48. doi: 10.1016/j.omto.2018.12.004. eCollection 2019 Mar 29. Mol Ther Oncolytics. 2018. PMID: 30666318 Free PMC article.
-
Presentation of hepatocellular antigens.Cell Mol Immunol. 2016 May;13(3):293-300. doi: 10.1038/cmi.2015.109. Epub 2016 Feb 29. Cell Mol Immunol. 2016. PMID: 26924525 Free PMC article. Review.
-
Listeria monocytogenes-derived listeriolysin O has pathogen-associated molecular pattern-like properties independent of its hemolytic ability.Clin Vaccine Immunol. 2013 Jan;20(1):77-84. doi: 10.1128/CVI.00488-12. Epub 2012 Nov 7. Clin Vaccine Immunol. 2013. PMID: 23136118 Free PMC article.
-
A Genetically Modified attenuated Listeria Vaccine Expressing HPV16 E7 Kill Tumor Cells in Direct and Antigen-Specific Manner.Front Cell Infect Microbiol. 2017 Jun 29;7:279. doi: 10.3389/fcimb.2017.00279. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28706878 Free PMC article.
References
-
- Ries LAG, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Eisner MP, Horner MJ, Howlader N, Hayat M, Hankey BF, Edwards BK (eds) (2006) SEER Cancer Statistics Review, 1975–2003. National Cancer Institute. Bethesda
-
- Marcus R, Imrie K, Belch A, Cunningham D, Flores E, Catalano J, Solal-Celigny P, Offner F, Walewski J, Raposo J, Jack A, Smith P. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105:1417–1423. doi: 10.1182/blood-2004-08-3175. - DOI - PubMed
-
- Jazirehi AR, Gan XH, De Vos S, Emmanouilides C, Bonavida B. Rituximab (anti-CD20) selectively modifies Bcl-xL and apoptosis protease activating factor-1 (Apaf-1) expression and sensitizes human non-Hodgkin’s lymphoma B cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther. 2003;2:1183–1193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

