Adjuvant therapeutic vaccination in patients with non-small cell lung cancer made lymphopenic and reconstituted with autologous PBMC: first clinical experience and evidence of an immune response
- PMID: 17868452
- PMCID: PMC2020458
- DOI: 10.1186/1479-5876-5-43
Adjuvant therapeutic vaccination in patients with non-small cell lung cancer made lymphopenic and reconstituted with autologous PBMC: first clinical experience and evidence of an immune response
Abstract
Background: Given the considerable toxicity and modest benefit of adjuvant chemotherapy for non-small cell lung cancer (NSCLC), there is clearly a need for new treatment modalities in the adjuvant setting. Active specific immunotherapy may represent such an option. However, clinical responses have been rare so far. Manipulating the host by inducing lymphopenia before vaccination resulted in a magnification of the immune response in the preclinical setting. To evaluate feasibility and safety of an irradiated, autologous tumor cell vaccine given following induction of lymphopenia by chemotherapy and reinfusion of autologous peripheral blood mononuclear cells (PBMC), we are currently conducting a pilot-phase I clinical trial in patients with NSCLC following surgical resection. This paper reports on the first clinical experience and evidence of an immune response in patients suffering from NSCLC.
Methods: NSCLC patients stages I-IIIA are recruited. Vaccines are generated from their resected lung specimens. Patients undergo leukapheresis to harvest their PBMC prior to or following the surgical procedure. Furthermore, patients receive preparative chemotherapy (cyclophosphamide 350 mg/m2 and fludarabine 20 mg/m2 on 3 consecutive days) for induction of lymphopenia followed by reconstitution with their autologous PBMC. Vaccines are administered intradermally on day 1 following reconstitution and every two weeks for a total of up to five vaccinations. Granulocyte-macrophage-colony-stimulating-factor (GM-CSF) is given continuously (at a rate of 50 microg/24 h) at the site of vaccination via minipump for six consecutive days after each vaccination.
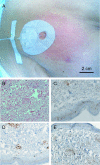
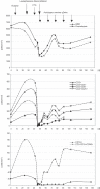
Results: To date, vaccines were successfully manufactured for 4 of 4 patients. The most common toxicities were local injection-site reactions and mild constitutional symptoms. Immune responses to chemotherapy, reconstitution and vaccination are measured by vaccine site and delayed type hypersensitivity (DTH) skin reactions. One patient developed positive DTH skin tests so far. Immunohistochemical assessment of punch biopsies taken at the local vaccine site reaction revealed a dense lymphocyte infiltrate. Further immunohistochemical differentiation showed that CD1a+ cells had been attracted to the vaccine site as well as predominantly CD4+ lymphocytes. The 3-day combination chemotherapy consisting of cyclophosphamide and fludarabine induced a profound lymphopenia in all patients. Sequential FACS analysis revealed that different T cell subsets (CD4, CD8, CD4CD25) as well as granulocytes, B cells and NK cells were significantly reduced. Here, we report on clinical safety and feasibility of this vaccination approach during lymphoid recovery and demonstrate a patient example.
Conclusion: Thus far, all vaccines were well tolerated. The overall trial design seems safe and feasible. Vaccine site reactions associated with infusion of GM-CSF via mini-pump are consistent with the postulated mechanism of action. More detailed immune-monitoring is required to evaluate a potential systemic immune response. Further studies to exploit homeostasis-driven T cell proliferation for the induction of a specific anti-tumor immune response in this clinical setting are warranted.
Figures


Similar articles
-
Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinoma.J Clin Oncol. 2003 Feb 15;21(4):624-30. doi: 10.1200/JCO.2003.03.091. J Clin Oncol. 2003. PMID: 12586798 Clinical Trial.
-
Immune modulations during chemoimmunotherapy & novel vaccine strategies--in metastatic melanoma and non small-cell lung cancer.Dan Med J. 2013 Dec;60(12):B4774. Dan Med J. 2013. PMID: 24355457 Review.
-
An open-label, prospective phase I/II study evaluating the immunogenicity and safety of a ras peptide vaccine plus GM-CSF in patients with non-small cell lung cancer.Lung Cancer. 2007 Oct;58(1):88-94. doi: 10.1016/j.lungcan.2007.05.003. Epub 2007 Jun 27. Lung Cancer. 2007. PMID: 17599645 Clinical Trial.
-
Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer.Cancer Gene Ther. 2006 Jun;13(6):555-62. doi: 10.1038/sj.cgt.7700922. Cancer Gene Ther. 2006. PMID: 16410826 Clinical Trial.
-
Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients.Ann Oncol. 2007 Feb;18(2):226-32. doi: 10.1093/annonc/mdl158. Epub 2006 Nov 20. Ann Oncol. 2007. PMID: 17116643 Review.
Cited by
-
Cancer immunotherapy: the role regulatory T cells play and what can be done to overcome their inhibitory effects.Curr Mol Med. 2009 Aug;9(6):673-82. doi: 10.2174/156652409788970670. Curr Mol Med. 2009. PMID: 19689294 Free PMC article. Review.
-
Differential effect on different immune subsets of neoadjuvant chemotherapy in patients with TNBC.J Immunother Cancer. 2020 Nov;8(2):e001261. doi: 10.1136/jitc-2020-001261. J Immunother Cancer. 2020. PMID: 33199511 Free PMC article.
-
Antigenic Essence: Upgrade of Cellular Cancer Vaccines.Cancers (Basel). 2021 Feb 12;13(4):774. doi: 10.3390/cancers13040774. Cancers (Basel). 2021. PMID: 33673325 Free PMC article. Review.
-
Disruption of TGF-beta signaling prevents the generation of tumor-sensitized regulatory T cells and facilitates therapeutic antitumor immunity.J Immunol. 2009 Sep 15;183(6):3682-9. doi: 10.4049/jimmunol.0900560. Epub 2009 Aug 19. J Immunol. 2009. PMID: 19692636 Free PMC article.
-
Inoculation site from a cutaneous melanoma patient treated with an allogeneic therapeutic vaccine: a case report.Front Immunol. 2015 Mar 30;6:144. doi: 10.3389/fimmu.2015.00144. eCollection 2015. Front Immunol. 2015. PMID: 25870600 Free PMC article.
References
-
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. - DOI - PMC - PubMed
-
- Salgia R, Lynch T, Skarin A, Lucca J, Lynch C, Jung K, Hodi FS, Jaklitsch M, Mentzer S, Swanson S, Lukanich J, Bueno R, Wain J, Mathisen D, Wright C, Fidias P, Donahue D, Clift S, Hardy S, Neuberg D, Mulligan R, Webb I, Sugarbaker D, Mihm M, Dranoff G. Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinoma. J Clin Oncol. 2003;21:624–630. doi: 10.1200/JCO.2003.03.091. - DOI - PubMed
-
- Nemunaitis J, Sterman D, Jablons D, Smith JW, Fox B, Maples P, Hamilton S, Borellini S, Lin A, Morali S, Hege K. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. J Natl Cancer Inst. 2004;96:326–331. - PubMed
-
- Levitsky HI. Augmentation of host immune responses to cancer: overcoming the barrier of tumor antigen-specific T-cell tolerance. Cancer J. 2000;6:S281–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

