MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets
- PMID: 17786230
- PMCID: PMC1952642
- DOI: 10.1172/JCI33099
MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets
Abstract
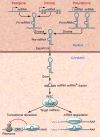
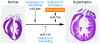
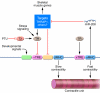
MicroRNAs act as negative regulators of gene expression by inhibiting the translation or promoting the degradation of target mRNAs. Recent studies have revealed key roles of microRNAs as regulators of the growth, development, function, and stress responsiveness of the heart, providing glimpses of undiscovered regulatory mechanisms and potential therapeutic targets for the treatment of heart disease.
Figures


Similar articles
-
MicroRNAs: novel regulators in cardiac development and disease.Cardiovasc Res. 2008 Sep 1;79(4):562-70. doi: 10.1093/cvr/cvn137. Epub 2008 May 29. Cardiovasc Res. 2008. PMID: 18511432 Review.
-
[Potential role of microRNAs in human diseases and the exploration on design of small molecule agents].Yao Xue Xue Bao. 2007 Nov;42(11):1115-21. Yao Xue Xue Bao. 2007. PMID: 18300464 Review. Chinese.
-
MicroRNA epigenetic alterations: predicting biomarkers and therapeutic targets in human diseases.Clin Genet. 2008 Oct;74(4):307-15. doi: 10.1111/j.1399-0004.2008.01075.x. Epub 2008 Aug 18. Clin Genet. 2008. PMID: 18713257 Review.
-
MicroRNAs: novel regulators in the hallmarks of human cancer.Cancer Lett. 2009 Nov 28;285(2):116-26. doi: 10.1016/j.canlet.2009.04.031. Epub 2009 May 22. Cancer Lett. 2009. PMID: 19464788 Review.
-
Toward microRNA-based therapeutics for heart disease: the sense in antisense.Circ Res. 2008 Oct 24;103(9):919-28. doi: 10.1161/CIRCRESAHA.108.183426. Circ Res. 2008. PMID: 18948630 Free PMC article. Review.
Cited by
-
Heart structure-specific transcriptomic atlas reveals conserved microRNA-mRNA interactions.PLoS One. 2013;8(1):e52442. doi: 10.1371/journal.pone.0052442. Epub 2013 Jan 2. PLoS One. 2013. PMID: 23300973 Free PMC article.
-
Transcription factor FoxO1, the dominant mediator of muscle wasting in chronic kidney disease, is inhibited by microRNA-486.Kidney Int. 2012 Aug;82(4):401-11. doi: 10.1038/ki.2012.84. Kidney Int. 2012. PMID: 22475820 Free PMC article.
-
MiR-30-regulated autophagy mediates angiotensin II-induced myocardial hypertrophy.PLoS One. 2013;8(1):e53950. doi: 10.1371/journal.pone.0053950. Epub 2013 Jan 9. PLoS One. 2013. PMID: 23326547 Free PMC article.
-
MicroRNA Regulatory Pathways in the Control of the Actin-Myosin Cytoskeleton.Cells. 2020 Jul 9;9(7):1649. doi: 10.3390/cells9071649. Cells. 2020. PMID: 32660059 Free PMC article. Review.
-
Horizontal transfer of microRNAs: molecular mechanisms and clinical applications.Protein Cell. 2012 Jan;3(1):28-37. doi: 10.1007/s13238-012-2003-z. Epub 2012 Feb 9. Protein Cell. 2012. PMID: 22314808 Free PMC article. Review.
References
-
- Hoffman J.I. Incidence of congenital heart disease: II. Prenatal incidence. Pediatr. Cardiol. 1995;16:155–165. - PubMed
-
- Rosamond W., et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. - PubMed
-
- Yang B., et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat. Med. 2007;13:486–491. - PubMed
-
- Zhao Y., et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–317. - PubMed
-
- van Rooij E., et al. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

