Unveiling the roles of autophagy in innate and adaptive immunity
- PMID: 17767194
- PMCID: PMC7097190
- DOI: 10.1038/nri2161
Unveiling the roles of autophagy in innate and adaptive immunity
Abstract
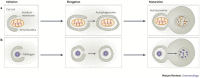
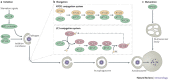
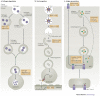
Cells digest portions of their interiors in a process known as autophagy to recycle nutrients, remodel and dispose of unwanted cytoplasmic constituents. This ancient pathway, conserved from yeast to humans, is now emerging as a central player in the immunological control of bacterial, parasitic and viral infections. The process of autophagy may degrade intracellular pathogens, deliver endogenous antigens to MHC-class-II-loading compartments, direct viral nucleic acids to Toll-like receptors and regulate T-cell homeostasis. This Review describes the mechanisms of autophagy and highlights recent advances relevant to the role of autophagy in innate and adaptive immunity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Autophagy in innate and adaptive immunity.Trends Immunol. 2005 Oct;26(10):523-8. doi: 10.1016/j.it.2005.08.003. Trends Immunol. 2005. PMID: 16099218 Review.
-
Autophagy in immunity and cell-autonomous defense against intracellular microbes.Immunol Rev. 2011 Mar;240(1):92-104. doi: 10.1111/j.1600-065X.2010.00995.x. Immunol Rev. 2011. PMID: 21349088 Free PMC article. Review.
-
Autophagy in CD4+ T-cell immunity and tolerance.Cell Death Differ. 2009 Jan;16(1):79-86. doi: 10.1038/cdd.2008.113. Epub 2008 Jul 18. Cell Death Differ. 2009. PMID: 18636073 Review.
-
Role of autophagy in immunity and autoimmunity, with a special focus on systemic lupus erythematosus.FASEB J. 2012 Apr;26(4):1400-12. doi: 10.1096/fj.11-194175. Epub 2012 Jan 12. FASEB J. 2012. PMID: 22247332 Review.
-
Links between autophagy, innate immunity, inflammation and Crohn's disease.Dig Dis. 2009;27(3):246-51. doi: 10.1159/000228557. Epub 2009 Sep 24. Dig Dis. 2009. PMID: 19786748 Free PMC article. Review.
Cited by
-
Persistent Activation of the P2X7 Receptor Underlies Chronic Inflammation and Carcinogenic Changes in the Intestine.Int J Mol Sci. 2024 Oct 10;25(20):10874. doi: 10.3390/ijms252010874. Int J Mol Sci. 2024. PMID: 39456655 Free PMC article. Review.
-
Is Autophagy a Friend or Foe in SARS-CoV-2 Infection?Viruses. 2024 Sep 20;16(9):1491. doi: 10.3390/v16091491. Viruses. 2024. PMID: 39339967 Free PMC article. Review.
-
Role of O-linked N-acetylglucosamine protein modification in oxidative stress-induced autophagy: a novel target for bone remodeling.Cell Commun Signal. 2024 Jul 10;22(1):358. doi: 10.1186/s12964-024-01734-3. Cell Commun Signal. 2024. PMID: 38987770 Free PMC article. Review.
-
Carboplatin restricts peste des petits ruminants virus replication by suppressing the STING-mediated autophagy.Front Vet Sci. 2024 May 15;11:1383927. doi: 10.3389/fvets.2024.1383927. eCollection 2024. Front Vet Sci. 2024. PMID: 38812563 Free PMC article.
-
Infectious factors in myocarditis: a comprehensive review of common and rare pathogens.Egypt Heart J. 2024 May 24;76(1):64. doi: 10.1186/s43044-024-00493-3. Egypt Heart J. 2024. PMID: 38789885 Free PMC article. Review.
References
-
- Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nature Rev. Mol. Cell. Biol. 2005;6:439–448. - PubMed
-
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell. 2004;6:463–477. - PubMed
-
- Deretic V. Autophagy in innate and adaptive immunity. Trends. Immunol. 2005;26:523–528. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

