Lymphocyte-dependent and Th2 cytokine-associated colitis in mice deficient in Wiskott-Aldrich syndrome protein
- PMID: 17764675
- PMCID: PMC2048975
- DOI: 10.1053/j.gastro.2007.07.010
Lymphocyte-dependent and Th2 cytokine-associated colitis in mice deficient in Wiskott-Aldrich syndrome protein
Abstract
Background & aims: Controversy exists as to whether patients with inflammatory bowel disease have an underlying immunodeficiency. We have focused on a murine model of the Wiskott-Aldrich syndrome, an immunodeficiency in which autoimmunity can manifest in the form of an inflammatory bowel disease-like illness. Wiskott-Aldrich syndrome protein (WASP) deficiency in mice results in similar clinical features. Herein, we characterized the colitis in WASP-deficient mice.
Methods: WASP-deficient mice were followed clinically and histologically. Immunologic studies were performed to determine the pathogenic cell population(s), the predominant cytokine expression pattern, and the role of cytokine(s) in colitis pathogenesis.
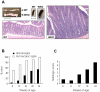
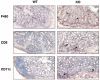
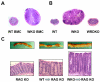
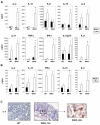
Results: All WASP-deficient mice develop colitis by 6 months of age. Lymphocytes are required for disease induction, and CD4(+) T cells from WASP-deficient mice are sufficient to induce disease in lymphocyte-deficient hosts. Lamina propria preparations from WASP-deficient mice demonstrated elevations in interferon-gamma, interleukin (IL)-4, and IL-13 levels but decreased IL-6 and no difference in IL-17 expression in comparison with wild-type controls. Treatment with neutralizing antibody to IL-4, but not to interferon-gamma, abrogated colitis development. However, mice deficient in both WASP and IL-4 showed no difference in histologic colitis scores at 24 weeks of age compared with WASP-deficient mice.
Conclusions: These results demonstrate a critical role for lymphocytes and a relative T helper 2 cytokine predominance in the colitis associated with WASP-deficient mice. This is the only model of colitis with elevated T helper 2 cytokines and aberrant natural regulatory T cell function and is unique in having a human disease counterpart with similar defects.
Figures



Similar articles
-
Wiskott-Aldrich syndrome protein deficiency in innate immune cells leads to mucosal immune dysregulation and colitis in mice.Gastroenterology. 2012 Sep;143(3):719-729.e2. doi: 10.1053/j.gastro.2012.06.008. Epub 2012 Jun 15. Gastroenterology. 2012. PMID: 22710191 Free PMC article.
-
Effects of Wiskott-Aldrich Syndrome Protein Deficiency on IL-10-Producing Regulatory B Cells in Humans and Mice.Scand J Immunol. 2015 Jun;81(6):483-93. doi: 10.1111/sji.12282. Scand J Immunol. 2015. PMID: 25728049
-
The Wiskott-Aldrich syndrome protein is required for the function of CD4(+)CD25(+)Foxp3(+) regulatory T cells.J Exp Med. 2007 Feb 19;204(2):381-91. doi: 10.1084/jem.20061338. Epub 2007 Feb 12. J Exp Med. 2007. PMID: 17296786 Free PMC article.
-
The role of WASp in T cells and B cells.Cell Immunol. 2019 Jul;341:103919. doi: 10.1016/j.cellimm.2019.04.007. Epub 2019 Apr 17. Cell Immunol. 2019. PMID: 31047647 Review.
-
Wiskott-Aldrich Syndrome at the nexus of autoimmune and primary immunodeficiency diseases.FEBS Lett. 2011 Dec 1;585(23):3710-4. doi: 10.1016/j.febslet.2011.10.031. Epub 2011 Oct 25. FEBS Lett. 2011. PMID: 22036785 Free PMC article. Review.
Cited by
-
Selected Cytokines and Metalloproteinases in Inflammatory Bowel Disease.Int J Mol Sci. 2023 Dec 22;25(1):202. doi: 10.3390/ijms25010202. Int J Mol Sci. 2023. PMID: 38203373 Free PMC article. Review.
-
Dysregulated inflammasome activity in intestinal inflammation - Insights from patients with very early onset IBD.Front Immunol. 2022 Nov 29;13:1027289. doi: 10.3389/fimmu.2022.1027289. eCollection 2022. Front Immunol. 2022. PMID: 36524121 Free PMC article. Review.
-
IL-17-Dependent Dysregulated Cutaneous Immune Homeostasis in the Absence of the Wiskott-Aldrich Syndrome Protein.Front Immunol. 2022 Feb 21;13:817427. doi: 10.3389/fimmu.2022.817427. eCollection 2022. Front Immunol. 2022. PMID: 35265075 Free PMC article.
-
Primary Immunodeficiencies Associated With Early-Onset Inflammatory Bowel Disease in Southeast and East Asia.Front Immunol. 2022 Jan 13;12:786538. doi: 10.3389/fimmu.2021.786538. eCollection 2021. Front Immunol. 2022. PMID: 35095863 Free PMC article.
-
Utilizing a reductionist model to study host-microbe interactions in intestinal inflammation.Microbiome. 2021 Nov 3;9(1):215. doi: 10.1186/s40168-021-01161-3. Microbiome. 2021. PMID: 34732258 Free PMC article.
References
-
- Sartor RB. Innate immunity in the pathogenesis and therapy of IBD. J Gastroenterol. 2003;38(Suppl 15):43–7. - PubMed
-
- Korzenik JR. Past and current theories of etiology of IBD: toothpaste, worms, and refrigerators. J Clin Gastroenterol. 2005;39:S59–65. - PubMed
-
- Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. - PubMed
-
- Mizoguchi A, Mizoguchi E, Bhan AK. Immune networks in animal models of inflammatory bowel disease. Inflamm Bowel Dis. 2003;9:246–59. - PubMed
-
- Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 AI050950/AI/NIAID NIH HHS/United States
- R01 AI050950-03/AI/NIAID NIH HHS/United States
- T32 DK007191/DK/NIDDK NIH HHS/United States
- DK64351/DK/NIDDK NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- DK64289/DK/NIDDK NIH HHS/United States
- P30 DK043351-17/DK/NIDDK NIH HHS/United States
- R03 DK074454/DK/NIDDK NIH HHS/United States
- T32DK007191/DK/NIDDK NIH HHS/United States
- R01 AI050950/AI/NIAID NIH HHS/United States
- R01 DK064351/DK/NIDDK NIH HHS/United States
- R01 AI081807/AI/NIAID NIH HHS/United States
- R01 DK055678/DK/NIDDK NIH HHS/United States
- HL59561/HL/NHLBI NIH HHS/United States
- AI50950/AI/NIAID NIH HHS/United States
- P01 HL059561/HL/NHLBI NIH HHS/United States
- R01 DK047677/DK/NIDDK NIH HHS/United States
- DK55678/DK/NIDDK NIH HHS/United States
- P01 HL059561-10/HL/NHLBI NIH HHS/United States
- DK47677/DK/NIDDK NIH HHS/United States
- K08 DK064289/DK/NIDDK NIH HHS/United States
- DK74454/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

