Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling
- PMID: 17728453
- PMCID: PMC6673128
- DOI: 10.1523/JNEUROSCI.2002-07.2007
Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling
Abstract
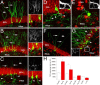
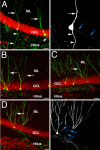
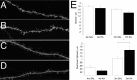
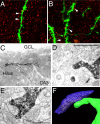
Seizure activity within the hippocampal circuitry not only affects pre-existing structures, but also dramatically increases the number of newborn granule cells. A retroviral strategy was used to label dividing cells and their progeny in the adult dentate gyrus and to analyze the impact of epileptic activity on adult-generated cells labeled before or after seizures. We show that epileptic activity led to dramatic changes in the neuronal polarity, migration, and integration pattern of newborn granule cells, depending on the time of birth in relation to the epileptic insult. Aberrant neurons were stably integrated into the dentate circuitry, and the consequences on hippocampal neurogenesis were long lasting. The data presented characterized the consequences of seizure-associated plasticity on adult neurogenesis leading to long-term structural changes in the hippocampal circuitry that might represent a pivotal component of the epileptic disease process.
Figures
Similar articles
-
Ablation of aberrant neurogenesis fails to attenuate cognitive deficit of chronically epileptic mice.Epilepsy Res. 2018 May;142:1-8. doi: 10.1016/j.eplepsyres.2018.03.004. Epub 2018 Mar 3. Epilepsy Res. 2018. PMID: 29524833
-
Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells.Exp Neurol. 2005 Dec;196(2):342-51. doi: 10.1016/j.expneurol.2005.08.010. Epub 2005 Oct 5. Exp Neurol. 2005. PMID: 16168988
-
Aberrant neurogenesis after stroke: a retroviral cell labeling study.Stroke. 2012 Sep;43(9):2468-75. doi: 10.1161/STROKEAHA.112.660977. Epub 2012 Jun 26. Stroke. 2012. PMID: 22738919
-
Seizure-induced neurogenesis: are more new neurons good for an adult brain?Prog Brain Res. 2002;135:121-31. doi: 10.1016/S0079-6123(02)35012-X. Prog Brain Res. 2002. PMID: 12143334 Review.
-
Adult neurogenesis in the intact and epileptic dentate gyrus.Prog Brain Res. 2007;163:529-40. doi: 10.1016/S0079-6123(07)63028-3. Prog Brain Res. 2007. PMID: 17765736 Review.
Cited by
-
Increased cell-intrinsic excitability induces synaptic changes in new neurons in the adult dentate gyrus that require Npas4.J Neurosci. 2013 May 1;33(18):7928-40. doi: 10.1523/JNEUROSCI.1571-12.2013. J Neurosci. 2013. PMID: 23637184 Free PMC article.
-
Interplay between DISC1 and GABA signaling regulates neurogenesis in mice and risk for schizophrenia.Cell. 2012 Mar 2;148(5):1051-64. doi: 10.1016/j.cell.2011.12.037. Cell. 2012. PMID: 22385968 Free PMC article.
-
Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy.Neuron. 2012 Sep 20;75(6):1022-34. doi: 10.1016/j.neuron.2012.08.002. Neuron. 2012. PMID: 22998871 Free PMC article.
-
Mechanisms regulating neuronal excitability and seizure development following mTOR pathway hyperactivation.Front Mol Neurosci. 2014 Mar 14;7:18. doi: 10.3389/fnmol.2014.00018. eCollection 2014. Front Mol Neurosci. 2014. PMID: 24672426 Free PMC article. Review.
-
Activin A is essential for neurogenesis following neurodegeneration.Stem Cells. 2009 Jun;27(6):1330-46. doi: 10.1002/stem.80. Stem Cells. 2009. PMID: 19489097 Free PMC article.
References
-
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. - PubMed
-
- Dalby NO, Mody I. The process of epileptogenesis: a pathophysiological approach. Curr Opin Neurol. 2001;14:187–192. - PubMed
-
- Dashtipour K, Wong AM, Obenaus A, Spigelman I, Ribak CE. Temporal profile of hilar basal dendrite formation on dentate granule cells after status epilepticus. Epilepsy Res. 2003;54:141–151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
