Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor
- PMID: 17723218
- PMCID: PMC2034442
- DOI: 10.1016/j.immuni.2007.07.010
Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor
Abstract
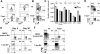
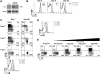
As acute infections resolve, effector CD8(+) T cells differentiate into interleukin-7 receptor(lo) (IL-7R(lo)) short-lived effector cells (SLECs) and IL-7R(hi) memory precursor effector cells (MPECs) capable of generating long-lived memory CD8(+) T cells. By using another SLEC marker, KLRG1, we found that KLRG1(hi) effector cells began appearing early during infection and were committed to downregulating IL-7R. Unlike IL-7R(hi) MPECs, KLRG1(hi) IL-7R(lo) SLECs relied on IL-15, but IL-15 could not sustain their long-term maintenance or homeostatic turnover. The decision between SLEC and MPEC fates was regulated by the amount of inflammatory cytokines (i.e., IL-12) present during T cell priming. According to the amount of inflammation, a gradient of T-bet was created in which high T-bet expression induced SLECs and low expression promoted MPECs. These results elucidate a mechanism by which the innate immune system sets the relative amounts of a lineage-determining transcription factor in activated CD8(+) T cells and, correspondingly, regulates their memory cell potential.
Figures





Comment in
-
CD8(+) T cell differentiation: choosing a path through T-bet.Immunity. 2007 Aug;27(2):180-2. doi: 10.1016/j.immuni.2007.08.003. Immunity. 2007. PMID: 17723210
Similar articles
-
Expression of IL-7Rα and KLRG1 defines functionally distinct CD8+ T-cell populations in humans.Eur J Immunol. 2019 May;49(5):694-708. doi: 10.1002/eji.201847897. Epub 2019 Mar 25. Eur J Immunol. 2019. PMID: 30883723 Free PMC article.
-
IL-12 signaling drives CD8+ T cell IFN-gamma production and differentiation of KLRG1+ effector subpopulations during Toxoplasma gondii Infection.J Immunol. 2008 May 1;180(9):5935-45. doi: 10.4049/jimmunol.180.9.5935. J Immunol. 2008. PMID: 18424713
-
IL-2 induction of Blimp-1 is a key in vivo signal for CD8+ short-lived effector T cell differentiation.J Immunol. 2014 Aug 15;193(4):1847-54. doi: 10.4049/jimmunol.1302365. Epub 2014 Jul 11. J Immunol. 2014. PMID: 25015830
-
Type-I IFN drives the differentiation of short-lived effector CD8+ T cells in vivo.Eur J Immunol. 2012 Feb;42(2):320-9. doi: 10.1002/eji.201142091. Epub 2011 Dec 20. Eur J Immunol. 2012. PMID: 22102057
-
Decisions on the road to memory.Adv Exp Med Biol. 2013;785:107-20. doi: 10.1007/978-1-4614-6217-0_12. Adv Exp Med Biol. 2013. PMID: 23456843 Review.
Cited by
-
Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection.Science. 2012 Nov 30;338(6111):1220-5. doi: 10.1126/science.1229620. Science. 2012. PMID: 23197535 Free PMC article.
-
Division-linked generation of death-intermediates regulates the numerical stability of memory CD8 T cells.Proc Natl Acad Sci U S A. 2012 Apr 17;109(16):6199-204. doi: 10.1073/pnas.1118868109. Epub 2012 Apr 2. Proc Natl Acad Sci U S A. 2012. PMID: 22474367 Free PMC article.
-
In vivo RNAi screens: concepts and applications.Trends Immunol. 2015 May;36(5):315-22. doi: 10.1016/j.it.2015.03.007. Epub 2015 Apr 27. Trends Immunol. 2015. PMID: 25937561 Free PMC article. Review.
-
Id2 influences differentiation of killer cell lectin-like receptor G1(hi) short-lived CD8+ effector T cells.J Immunol. 2013 Feb 15;190(4):1501-9. doi: 10.4049/jimmunol.1200750. Epub 2013 Jan 16. J Immunol. 2013. PMID: 23325888 Free PMC article.
-
Distinct mechanisms mediate naive and memory CD8 T-cell tolerance.Proc Natl Acad Sci U S A. 2012 Dec 26;109(52):21438-43. doi: 10.1073/pnas.1217409110. Epub 2012 Dec 10. Proc Natl Acad Sci U S A. 2012. PMID: 23236165 Free PMC article.
References
-
- Agnello D, Lankford CS, Bream J, Morinobu A, Gadina M, O'Shea JJ, Frucht DM. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J Clin Immunol. 2003;23:147–161. - PubMed
-
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. - PubMed
-
- Bachmann MF, Beerli RR, Agnellini P, Wolint P, Schwarz K, Oxenius A. Long-lived memory CD8+ T cells are programmed by prolonged antigen exposure and low levels of cellular activation. Eur J Immunol. 2006;36:842–854. - PubMed
-
- Bachmann MF, Schwarz K, Wolint P, Meijerink E, Martin S, Manolova V, Oxenius A. Cutting edge: distinct roles for T help and CD40/CD40 ligand in regulating differentiation of proliferation-competent memory CD8+ T cells. J Immunol. 2004;173:2217–2221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

