Paf1 complex homologues are required for Notch-regulated transcription during somite segmentation
- PMID: 17721442
- PMCID: PMC1973952
- DOI: 10.1038/sj.embor.7401045
Paf1 complex homologues are required for Notch-regulated transcription during somite segmentation
Abstract
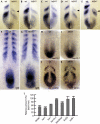
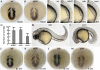
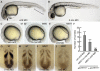
Members of the yeast polymerase-associated factor 1 (Paf1) complex, which is composed of at least five components (Paf1, Rtf1, Cdc73, Leo1 and Ctr9), are conserved from yeast to humans. Although these proteins have been implicated in RNA polymerase II-mediated transcription, their roles in vertebrate development have not been explained. Here, we show that a zebrafish mutant with a somite segmentation defect is deficient in rtf1. In addition, embryos deficient in rtf1 or ctr9 show abnormal development of the heart, ears and neural crest cells. rtf1 is required for correct RNA levels of the Notch-regulated genes her1, her7 and deltaC, and also for Notch-induced her1 expression in the presomitic mesoderm. Furthermore, the phenotype observed in rtf1-deficient mutants is enhanced by an additional deficiency in mind bomb, which encodes an effector of Notch signalling. Therefore, zebrafish homologues of the yeast Paf1 complex seem to preferentially affect a subset of genes, including Notch-regulated genes, during embryogenesis.
Figures

Similar articles
-
tortuga refines Notch pathway gene expression in the zebrafish presomitic mesoderm at the post-transcriptional level.Dev Biol. 2005 Nov 15;287(2):225-36. doi: 10.1016/j.ydbio.2005.07.032. Epub 2005 Oct 19. Dev Biol. 2005. PMID: 16236276
-
Analysis of her1 and her7 mutants reveals a spatio temporal separation of the somite clock module.PLoS One. 2012;7(6):e39073. doi: 10.1371/journal.pone.0039073. Epub 2012 Jun 18. PLoS One. 2012. PMID: 22723933 Free PMC article.
-
Hairy/E(spl)-related (Her) genes are central components of the segmentation oscillator and display redundancy with the Delta/Notch signaling pathway in the formation of anterior segmental boundaries in the zebrafish.Development. 2002 Jun;129(12):2929-46. doi: 10.1242/dev.129.12.2929. Development. 2002. PMID: 12050140
-
[Wave and somite segmentation: a view from zebrafish genetic analyses].Tanpakushitsu Kakusan Koso. 2002 Dec;47(15):2017-23. Tanpakushitsu Kakusan Koso. 2002. PMID: 12486933 Review. Japanese. No abstract available.
-
Oscillators and the emergence of tissue organization during zebrafish somitogenesis.Trends Cell Biol. 2007 Dec;17(12):593-9. doi: 10.1016/j.tcb.2007.09.005. Epub 2007 Nov 7. Trends Cell Biol. 2007. PMID: 17988868 Review.
Cited by
-
The Roles of the Paf1 Complex and Associated Histone Modifications in Regulating Gene Expression.Genet Res Int. 2011;2011:707641. doi: 10.4061/2011/707641. Genet Res Int. 2011. PMID: 22408743 Free PMC article.
-
Distinct role of subunits of the Arabidopsis RNA polymerase II elongation factor PAF1C in transcriptional reprogramming.Front Plant Sci. 2022 Sep 29;13:974625. doi: 10.3389/fpls.2022.974625. eCollection 2022. Front Plant Sci. 2022. PMID: 36247629 Free PMC article.
-
Drosophila CG2469 Encodes a Homolog of Human CTR9 and Is Essential for Development.G3 (Bethesda). 2016 Dec 7;6(12):3849-3857. doi: 10.1534/g3.116.035196. G3 (Bethesda). 2016. PMID: 27678520 Free PMC article.
-
The PAF1 complex differentially regulates cardiomyocyte specification.Dev Biol. 2011 May 1;353(1):19-28. doi: 10.1016/j.ydbio.2011.02.011. Epub 2011 Feb 19. Dev Biol. 2011. PMID: 21338598 Free PMC article.
-
RNA helicase DDX21 mediates nucleotide stress responses in neural crest and melanoma cells.Nat Cell Biol. 2020 Apr;22(4):372-379. doi: 10.1038/s41556-020-0493-0. Epub 2020 Mar 30. Nat Cell Biol. 2020. PMID: 32231306 Free PMC article.
References
-
- Gajewski M, Sieger D, Alt B, Leve C, Hans S, Wolff C, Rohr KB, Tautz D (2003) Anterior and posterior waves of cyclic her1 gene expression are differentially regulated in the presomitic mesoderm of zebrafish. Development 130: 4269–4278 - PubMed
-
- Holley SA, Takeda H (2002) Catching a wave: the oscillator and wavefront that create the zebrafish somite. Semin Cell Dev Biol 13: 481–488 - PubMed
-
- Horikawa K, Ishimatsu K, Yoshimoto E, Kondo S, Takeda H (2006) Noise-resistant and synchronized oscillation of the segmentation clock. Nature 441: 719–723 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

